Research
The O’Connor group explores the enzyme based biosynthetic pathways that generate structurally complex and clinically useful natural products. Our goals are to use the pathways to produce novel products, understand the mechanisms of the individual enzymes, to modulate the substrate specificity of the enzymes and to discover new enzymes involved in natural product biosynthesis. A strong emphasis is placed on plant derived compounds. Although plants produce many pharmaceutically important, structurally complex products, relatively little is known about plant metabolic pathways.
We take a multi-disciplinary approach to address these questions: protein expression, plant cell culture, molecular biology, enzymology, assay design, natural product isolation and chemical synthesis are key components of our research.
Enzyme mechanism and engineering.
Alkaloid Biosynthetic Pathways
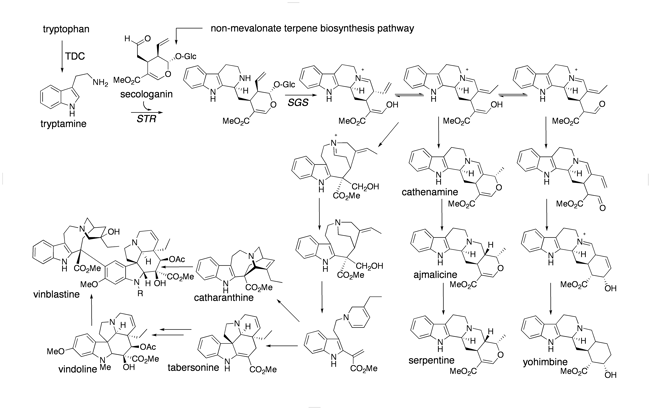
Alkaloids– nitrogen containing natural products– comprise some of the most structurally interesting and medicinally useful molecules found in nature. However, alkaloid biosynthesis is not well understood compared to other classes of natural products. We are focusing on the terpene indole alkaloid biosynthetic pathway from periwinkle (Catharanthus roseus). This pathway is responsible for the biosynthesis of thousands of structurally complex and clinically used compounds, such as vinblastine, ajmaline and quinine.
Biosynthesis of major terpene indole alkaloids in C. roseus.
Natural Product Biosynthesis
Many antibiotics, anti-tumor drugs and other pharmaceuticals are produced by bacteria, fungi or plants. These molecules are often harvested from the environment for clinical purposes. Unfortunately, isolation of compounds from the environment can also be an expensive and low yielding process. Furthermore, isolation procedures provide limited opportunities to modify the chemical and biological properties of the natural product.
Understanding the enzymes that catalyze natural product synthesis may enable production in more tractable host organisms and may also facilitate reprogramming of biosynthetic pathways to produce "unnatural" natural products with improved pharmacological activities.
C. roseus (Madagascar periwinkle) cell suspension culture displaying monoterpene indole alkaloid production.

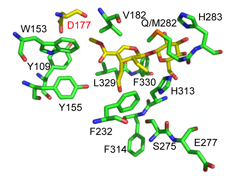

Directed biosynthesis of new alkaloids.