|
SP753 Project:
TB Drug Sensitivity Testing Kit |
Created by: Carmen Berg, Zahra Kanji, Kakul Srivastava
BACKGROUND INFORMATION
What is TB?
How big of a problem is
Tuberculosis today?
What prevention methods are available?
I thought TB was curable.
Why is it still so wide-spread?
What is being done to combat
poor treatment compliance?
Definitions
What is TB?
TB (Tuberculosis) is a
disease caused by a bacterium (germ) that is spread through the air usually
when a person who has the disease coughs, sneezes or breathes.
How big of a problem is Tuberculosis
today?
Among infectious diseases,
tuberculosis is the leading killer of adults in the world today. It poses
a serious challenge to international public health work. Currently, one
third of the world's population is infected with the TB bacillus. In the
next decade it is estimated that 300 million more people will become infected,
90 million people will develop the disease, and 30 million people will
die from it. TB is especially devastating in developing countries, where
it accounts for more than a quarter of all preventable adult deaths.
What prevention methods are
available?
Because TB is very infectious, it is important for the diagnosis
and treatment of the disease to be wide-spread in order for it to be effective.
Research is being done to develop a quick dip-stick test, but until that
project is complete, screening for the disease is done by a sputum smear
test.
I thought TB was
curable. Why is it still so wide-spread?
TB disease can be treated
and cured with medication. TB treatment starts with at least four drugs
which requires the patient to take about 12 pills a day for an extended
period (about 6 months). Some of the drugs used are Isoniazid (INH)
and Rifampin (Rifampin). Skipping medication or stopping might
lead TB to become contagious again. Patients often do not follow
through with the entire course of treatment. Failure to complete the treatment
leads to reinfection by multi-drug resistant (MDR) TB (which has a cure
rate of only about 20%). If it were possible to make the treatment easier
to complete (perhaps a time-release injectible system, or a transdermal
system), the number of MDR cases would decrease greatly.
What is being done to
combat poor treatment compliance?
Poor compliance
with the current drug system has sparked programs like DOT (Direct Observed
Treatment) in developing countries. DOT is a system which insists upon
checking that every patient takes every pill they need to. Even though
this system gets the job done, it is a very tedious one. The number of
patients infected by TB times the number of pills they must take adds to
a staggering number.
Definitions
1. MDR-TB - (Multi-drug Resistant Tuberculosis)
TB that is resistant to at least two of the main drugs
used to treat TB (INH and Rifampin). TB can become resistant if the
treatment isn't treated long enough, doesn't receive the right drugs or
isn't medicated properly.
2. Isoniazid (INH) - Oral tablet to treat TB.
Daily dosage of 300 mg. Side effects: liver problems, neuropathy
(pain or tingling in hands and feet), fever or rash. Drug tips: take
food to reduce stomach problems, take vitamin B6 to reduce neuropathy,
do not drink alcohol (increases liver problems).
3. Rifampin (Rifampin) - Oral tablet to treat TB.
Daily dosage of 600 mg. Side effects: upset stomach, heart
burn, nausea, headache, drowsiness or dizziness. Drug tips:
take medication on an empty stomach on hour before a meal or at least two
hours after.
PROJECT DESIGN
Problems and Solutions
Protocol
Implementing TB treatments in developing countries
is a challenge in itself because medical facilities in third world countries
are poorly equipped. Many things that we take for granted here are
not readily available there, such as electricity and clean water.
We tried to keep these limitations in mind in designing ways to improve
TB treatment.
Our research team, during the spring
semester at MIT, thought of a few other alternative methods to the DOT
system. These are listed below with the specific problem were were trying
to solve and our solutions to them.
|
PROBLEMS
|
SOLUTIONS
|
| The drugs have a sour taste to them. |
Mix drugs with sugar substance to make pills taste more
like candy. |
| The drugs are hard to swallow. |
Administer drugs using transdermal patches. |
| The treatment is long and has poor follow through. |
Introduce a reward at the end of each month |
| It is hard to remember to take the drugs daily. |
1. Create a handy dispenser, easy to carry around
in a visible area.
2. Entice companies such as Coke to run the DOT
system. Take required pill, get a free bottle of Coke.
3. Create slow release, once-a-day formulations. |
Other problems that we thought were the cause of the
poor compliance were the supply of pills at local clinics and the general
public's belief in Western medicine and pills.
After researching all the above options, we realized
that many of the solutions were not feasible in the near future.
For example, novel drug delivery systems for TB drugs are not perfected
for usage currently. Although none of these solutions are feasible,
we found another way to address the TB problem in developing countries:
creating a TB drug sensitivity testing kit.
The TB drug sensitivity testing kit could be useful
in a variety of situations. By finding out what TB drugs a person
is susceptible to, the use of unnecessary antibiotics can be curbed and
MDR can be reduced. In addition, this test can be used as a diagnostic
tool in areas where widespread resistance to a particular drug is suspected.
Protocol for TB Drug Sensitivity
Kit in Developing Countries
This protocol is a modified version of TB sensitivity
testing protocol in the following sources:
Public Health Mycobacteriology: A Guide
for the Level III Laboratory. US Department of Health and
Human Services. 1985.
Antimycobacterial Susceptibility Testing. The National
Committee for Clinical Laboratory Standards. 1990.
Protocol
Chemicals needed: Sodium hydroxide, sodium citrate
dihydrate, N-acetyl L-cysteine (NALC), Middlebrook 7H-10 basal medium,
distilled water, glucose and TB drugs to be tested.
To Isolate Specimen:
-
Make a 4% Sodium Hydroxide solution by adding 4.0 g
of NaOH to 100 ml of distilled water in a 150 ml bottle.
-
Make a 2.9% sodium citrate dihydrate solution by adding 2.9
g of sodium citrate dihydrate to 100 ml of distilled water in another 150
ml bottle.
-
Mix 25 ml of each solution together. This can be stored
if capped tightly. When the solution is ready to be used, add 0.25
g of N-acetyl L-cysteine (NALC). Once this is added, the solution
can be stored for only 24 hours.
-
Transfer up to 10 ml of sputum from patient into a 50 ml
plastic centrifuge tube with screw lid. Add an equivalent volume
of the NaOH/sodium citrate/NALC solution.
-
Mix the contents of the centrifuge tube with the lid tightly
capped for 5-20 seconds, avoid extreme agitation.
-
Let the tube stand for 15 minutes at room temperature for
decontamination.
-
Pour into graduated cylinder and dilute to the 50 ml mark
with distilled water. Pour back into centrifugation tube and mix
by swirling or inversion.
-
Centrifuge the mixture at 300 rpm for 15 minutes. Pour
off supernatant into a splash proof container and decontaminate lid of
tube by swabbing it with phenol. Recap tube.
-
Resuspend sediment in 1-2 ml of distilled water.
-
Mix and then make dilutions as follows:
-
Take 1 ml of resuspended solution and add 9 ml distilled
water. This is the 1/10 dilution. Then take 1 ml of this 1/10
dilution and add 9 ml distilled water. This is the 1/100 dilution.
Then take 1 ml of the 1/100 dilution and add 9 ml of distilled water.
This is the 1/1000 dilution.
-
The solutions are now ready to be plated on agar plates.
To make agar plates (this is using Middlebrook 7H-10 Medium--other
types of medium
um may be used, as long as they are compatible with mycobacterial
culture).
-
Suspend 3.6 g of Middlebrook 7H-10 basal medium in 180 ml
of freshly distilled water and add 1.0 of reagent grade glycerol.
-
Swirl base into suspension and sterilize by filtering through
a 0.22 um filter. Then add 20 ml of OADC enrichment.
-
Add the required amount of drug (see chart).
-
Plate on a quadrant of a petri dish.
-
Allow to solidify at room temperature without exposing it
to daylight.
-
Repeat for each drug condition being used.
After plates are created, place three drops of diluted solution
on each quadrant of drug-imbedded 7H-10 medium. Repeat for each dilution.
|
DRUGS
|
CONCENTRATIONS IN 7H-10 (ug/ml)
|
AMOUNT OF DRUG ADDED TO 200 ML SOLUTION (mg)
|
|
Isoniazid
|
0.2
|
0.04
|
|
Streptomycin
|
2.0
|
0.4
|
|
Ethambutol
|
5.0
|
1.0
|
|
Rifampin
|
1.0
|
0.2
|
|
p-Aminosalicyclic acid
|
2.0
|
0.4
|
|
Kanamycin
|
5.0
|
1.0
|
|
Ethionamide
|
5.0
|
1.0
|
|
Capreomycin
|
10.0
|
2.0
|
|
D-Cycloserine
|
30.0
|
6.0
|
|
Pyrazinamide
|
25.0
|
5.0
|
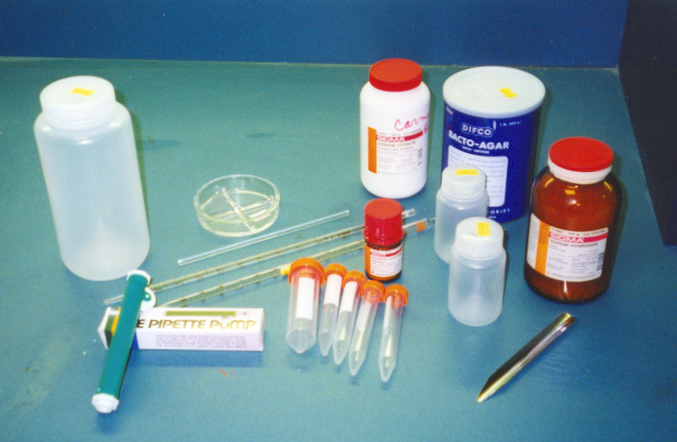
Examples of equipment and chemicals in TB Testing
Kit.
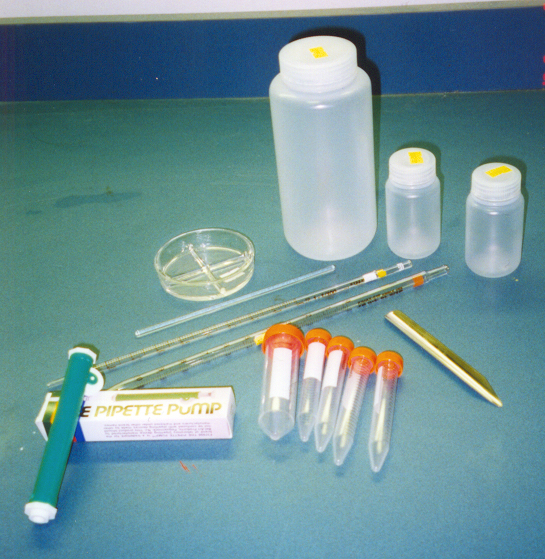
Depending on the resources and availability of supplies in
the area, either disposable or glass equipment can be used.
In designing this protocol, we assumed the following
resources would be available to the users of the kit: centrifuge,
sterile environment, incubator (if possible), refrigerator.
RECOMMENDATIONS
Our protocol design is fairly basic. Due to the
fact that TB is such a serious problem today, we felt that this kit
may help the current situation while our other ideas may provide future
solutions. Future projects such as an easy drug delivery system should
still be pursued that will increase the cure rate of TB in developing
nations.
Our protocol is based on research and current methods
used in the US. It has not been tested using TB mycobacteria
and thus cannot be guaranteed to provide accurate results. This protocol
should be tested in a laboratory to determine if it accurately determines
drug susceptibility.
This page is maintained by Amy Smith,
mmadinot@mit.edu

