(a) 268.8 K and 4.625
(b) at 90o (horizontal) 282.6 K and 4.863
(c) at 180o (vertical downward) 296.4 K and 5.1
| Answers to Selected Problems |
|
Chapter 3 Chapter 4 Chapter 5 Chapter 6 Chapter 7 Chapter 8 |
Chapter 9 Chapter 10 Chapter 11 Chapter 12 Chapter 13 Chapter 14 |
Chapter 15 Chapter 16 Chapter 17 Chapter 18 Chapter 19 |
3.1
(a) 268.8 K and 4.625![]() 105
Pa
105
Pa
(b) at 90o (horizontal) 282.6 K and 4.863![]() 105
Pa
105
Pa
(c) at 180o (vertical downward) 296.4 K and
5.1![]() 105
Pa
105
Pa
3.2
(a) ![]() Q/
Q/![]() t
= 3.51
t
= 3.51![]() 108
J/hr [contractor pays]
108
J/hr [contractor pays]
(b) ![]() Q/
Q/![]() t
= 3.23
t
= 3.23![]() 108
J/hr [contractor collects fee]
108
J/hr [contractor collects fee]
3.5
(a) LA = 0.4352 m, LB
=
0.0218 m, TA = TB = 311 K, PA
=
PB
=
7 bar
(b) Same as (a)
(c) There is no real solution given the constraints,
we must specify more about the expansion (or compression) of A or B. For
example, if A expands adiabatically, PA = PB
=
6.68 bar, TA = 263.6 K, TB = 881.3
K, LA = 0.3917 m, and LB = 0.0655 m.
3.12
(a) After 6 s, T = 349.5 K, P = 1.139![]() 105
Pa in the bulge.
105
Pa in the bulge.
(b) After 3 s, T = 454.6 K, P = 6.26![]() 103
Pa in the large tank.
103
Pa in the large tank.
4.1
For the minimum condition TA(final)
= 132 K and for the maximum condition TA(final) =617
K, these extrema require using the object with the lowest mass x heat capacity
product.
4.2
W = 9.807y in J/kg evaporated, for typical
values of ambient dry bulb and wet bulb temperatures of 300 K and 280 K
respectively, y = 16.8 km!
4.3
(a) ![]() B
= 0.166 mol/s,-
B
= 0.166 mol/s,-![]() C
= 0.833 mol/s
C
= 0.833 mol/s
(b) TD = 299.1K
(c) W = -26.4 J/s
(d) ![]() S=
13.3 J/K
S=
13.3 J/K
(e) Same as (d).
(f) ![]() =
-4000 J/s
=
-4000 J/s
4.4 W = - 1.11![]() 104
J, Tf = 288.7 K, Pf = 0.54 bar
104
J, Tf = 288.7 K, Pf = 0.54 bar
4.6
(a) 21.6 s
(b) 391.8 K and 2.2 bar at 10 s
(c) ![]() Sgas=
4.93
Sgas=
4.93![]() 103
J/K =
103
J/K = ![]() Suniverse
Suniverse
(d) ![]() S(gas
in tank) = -813.6 J/K,
S(gas
in tank) = -813.6 J/K,
-----![]() S(gas
vented) = +2020 J/K,
S(gas
vented) = +2020 J/K,
-----![]() S(surroundings)
= +1203 J/K
S(surroundings)
= +1203 J/K
(e) Wmin = +2.83![]() 105
J
105
J
4.7
(a) ![]() =
2400 W, T = 327.7 K, and Q = 22,200 J after 10 s
=
2400 W, T = 327.7 K, and Q = 22,200 J after 10 s
(b) T = 1750 K at t = 57.7 s with all stored
work consumed.
4.9
(a) Wnet = - 3.17![]() 106
J
106
J
4.12
(a) T2 = 227.9 K, T1
= 392.9 K (2) after venting (1) before venting
(b) T2 = 211.1 K, T1=
363.9 K
(c) W = 2547 J/mol
(d) Wmax = 1.38![]() 105
J/kg
105
J/kg
4.13
(a) ![]() S
= -1.38
S
= -1.38![]() 103
J/K hr
103
J/K hr
(b) ![]() S
= 2 J/K hr
S
= 2 J/K hr
4.16
(a) Case (1) 1.98![]() 10-2
kWhr; (2) 2.78
10-2
kWhr; (2) 2.78![]() 10-2
kWhr; (3) 2.16
10-2
kWhr; (3) 2.16![]() 10-2 kWhr;
(4) 2.78
10-2 kWhr;
(4) 2.78![]() 10-2
kWhr
10-2
kWhr
(b) Case (1) 420 K, 42 J/K; (2) 300 K, 0 J/K; (3) 300
K, 73.9 J/K; (4) 300 K, 0 J/K
4.18 W = 5.23![]() 103
J = -Q
103
J = -Q
4.22
(a) P = 6.35![]() 104
Pa
104
Pa
(b) T = 272.8 K
(c) P = 3.68![]() 104
Pa with 2 pumps
104
Pa with 2 pumps
(d) P = 1.91![]() 104
Pa and T
104
Pa and T![]()
(e) Power (minimum) = 20.9 W
4.24
(a) Wmax= -198.76 kJ/kg (Carnot + expansion
work)
(b) Wp = ideal pump work (estimated
to include PE for lifting water and for the compression of gas space in
storage tanks)
4.30
(a) ![]() =
6.38
=
6.38![]() 106 J/s
106 J/s
(b)![]() =
0.90 = (actual power)/(maximum power)
=
0.90 = (actual power)/(maximum power)
(c) no, the maximum power outputs are the same
5.2
(a) 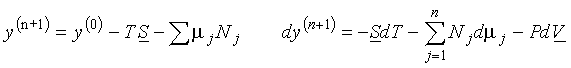
(b) 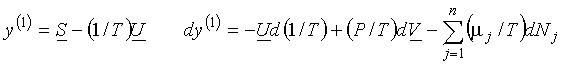
5.4
(a) ![]()
(b) ![]()
(c) U
(d) ![]()
5.7
(a) ![]()
(b) ![]()
5.10 Tf = 1087 K
5.12 ![]()
5.21 Vc = ![]() RT
= (Cp/Cv) RT » 330 m/s
(use mass units for R with molecular weight) distance at 2 s »
660 m
RT
= (Cp/Cv) RT » 330 m/s
(use mass units for R with molecular weight) distance at 2 s »
660 m
5.22
(a) ![]()
5.23
(b) No, the ratio as shown in Problem 5.17 is expressed
in terms of PVT properties and an isentropic derivative which in
turn requires non-PVT property information specifically related
to the temperature dependence of energy stored in U.
5.24
(a) ![]()
(b) ![]()
(c) ![]()
(d)![]()
(e) ![]()
5.25
(a) ![]()
(b) 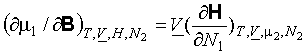
(c) Yes, using the Gibbs-Duhem relationship:
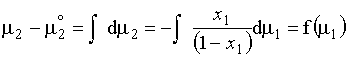
6.2
![]()
thus, ![]() at equilibrium
at equilibrium
6.3
-![]() from chemical equilibrium criteria, intersections define equilibrium states.
from chemical equilibrium criteria, intersections define equilibrium states.
6.4
(a) ![]()
----![]()
-----![]()
-----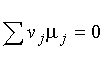
6.5
(a)![]() ,
, ![]()
(b) T = 366.5 K, PA,1 = PA,2
= 2 bar, PB,2 = 1 bar
NB,1
= 0 NB,2 = 1 mole NA,1 +
NA,2
= 4 moles
(c) P1 = 2 bar, N1
= 5 moles; V1 = 2.5 RT/105 =
0.0752 m3 with T = 367 K and no gas on side
2.
6.7
Fraction evaporated ![]() 0.227
0.227
![]()
![]()
![]()
7.1
if ANN = 0 then AVV
= 0 = ANN /V 2
7.3
T = 55.1 K
7.6
for ![]() = 2.5
= 2.5
| xA | xB |
| 0.1 | 0.286, 0.614 |
| 0.2 |
0.4 (critical pt.)
|
| 0.3 |
no solution
|
| 0.4 | 0.2, 0.4 (critical pts.) |
| 0.5 |
0.175, 0.325
|
| 0.6 |
0.085, 0.28
|
7.11
![]()
![]()
8.4
At 365.8 K, 16.5 bar
![]()
8.5
At T = 319.4 K
| Pr | KH/KS | Z = PV/RT |
| 0.1 | 0.100 | 0.967 |
| 0.2 | 0.194 | 0.932 |
| 0.4 | 0.369 | 0.862 |
| 1.0 | 0.798 | 0.598 |
| 4.0 | 0.426 | 0.547 |
| 10.0 | -0.296 | 1.108 |
8.9
Using an isenthalpic expansion across an insulated value
to 1 bar, 0.236 kg of dry ice could
be produced per kg of CO2 drawn from the cylinder.
For an isentropic expansion, 0.41 kg dry ice/kg of CO2 is possible.
8.10
(a) Tf = 379.3 K at 48.3 bar
(b) W = - 9.1![]() 107
J
107
J
8.14
TB,f|![]() 256
to 257 K, PB,f|
256
to 257 K, PB,f|![]() 21.5
bar, xg = 0.63 (fraction vapor)
21.5
bar, xg = 0.63 (fraction vapor)
8.15
(a) Power = 508 kW
(b) Power = 516 kW
8.30
-![]() = 90.9 kW and-
= 90.9 kW and-![]() = 68.3 kW
= 68.3 kW
8.31
9.1
(a) -31.36 kJ/mole water added to acid at the start;
-61.1 kJ/mole and added to water
at the start
(b) maximum heat load occurs at the start
(c) total Q = - 28 kJ/mol acid = -11.2 kJ/mol
of solution same for both cases
9.2
(b) Q = - 3.86 ![]() 105
J
105
J
(c)![]()
![]()
(d)![]()
9.3
mole % NH3 = 51.37 %
9.6
W = 326 J
9.7
![]()
w = water and s = salt (NaCl)
9.8
(a) Wmin = -1069 J (PREOS)
(b) Wmin = -1812 J (ideal gas mixture)
9.9
![]()
9.11
| x salt (wt fraction) | ||
| 0.01 | 7.2 | 8.5 |
| 0.05 | 42.2 | 43.9 |
| 0.10 | 90.5 | 91.1 |
| 0.15 | 156.6 | 142.0 |
| 0.20 | 242.0 | 197.0 |
| 0.25 | 355.0 | 257.0 |
9.13
![]()
![]()
9.14
(a) ![]()
(b) At -85oC (188 K), there are
two phases in equilibrium at xEtOH = 0.07 and
0.35.
9.20
![]() =
675 W
=
675 W
![]()
9.21
(a) ![]() 1.32
1.32![]() 105
W
105
W
(b) See Section 14.4 for details, information provided
in part (a) is not sufficient, ideal gas state heat capacities and a mixture
PVTN
EOS are needed.
9.27
![]()
10.2
H - Ho = PV - RT - a/V
10.3
![]()
![]()
![]()
![]()
10.4
![]()
10.6 ![]()
![]()
![]()
![]()
10.7
b = ![]()
a = ![]()
10.9
(a) ![]()
(b) ![]()
(c) for T/Tc = 1.00001,
![]()
10.10
![]()
11.4
(a) ![]()
(b)![]()
(c) for only positive values of ![]() ,
a large number of values
,
a large number of values ![]()
exist as a function of xi that yield
extrema, e.g.
for x1 = 0.2
| 0 | 3.236 |
| 0.500 | 1.699 |
| 1.000 | 1.000 |
| 1.500 | 0.586 |
| 2.000 | 0.209 |
| 2.118 | 0 |
11.5
for ![]()
for ![]()
11.7
(c) fraction of solution precipitated = 0.0067
11.8
for 2-suffix Margules Tc = w/2
k
11.9
for quasi-chemical model Tc = w/2.23
k
12.3
(a) selected values of ![]() are
given below for LiCl
are
given below for LiCl
| - |
- |
|
| 0.1 | 0.9651 | 0.9653 |
| 0.01 | 0.9032 | 0.9044 |
| 0.1 | 0.7850 | 0.7927 |
| 1.0 | 0.7636 | 0.7758 |
| 5.0 | 1.9586 | 2.0222 |
| 10.0 | 7.9948 | 11.0827 |
(b) ![]()
given that s = 9 mol/kg for pure LiCl in H2O.
Using the Meissner model for ![]() predictions for both pure and mixed electrolyte.
predictions for both pure and mixed electrolyte.
Ksp![]() (9)2
(~6)2
= 2916 for pure LiCl at 25oC
(9)2
(~6)2
= 2916 for pure LiCl at 25oC
![]()
Iterating
s*=2.4 and ![]() 3H for mixed LiCl and HCl at 25oC
3H for mixed LiCl and HCl at 25oC
(c) Difficult to say without more information on how
much Na2SO4
influences ![]() for
LiCl. For example is the effect on
for
LiCl. For example is the effect on ![]() large enough to compensate for the Cl- common ion effect?
large enough to compensate for the Cl- common ion effect?
12.8
Some representative values for ![]()
molality ![]() (NaCl
or LiCl)
(NaCl
or LiCl)![]() (CuCl2)
(CuCl2)
0.1
0.96
0.55
1.0
0.30
0.18
10.0
0.096
0.055
At T = 300 oC ![]() 22.3
so multiply above values by (22.3
22.3
so multiply above values by (22.3![]() 573)/(78.5)(298) = 0.74
573)/(78.5)(298) = 0.74
12.10
I = 0.546 or m = I / 3 =
0.182 molal
13.2
Using Joback’s method with Tb = 225.5K
(exp. value)
-Tc
= 364 K, Pc = 46.7 bar, and ![]() =
0.1606
=
0.1606
-![]() = -2.83 + 0.269 T = 3.15
= -2.83 + 0.269 T = 3.15![]() 10-4
T
2
+ 4.2
10-4
T
2
+ 4.2![]() 10-8
T
3
10-8
T
3
13.3
Tc = 663.5 K, Pc =
32.21 bar,
Vc
= 429.5 cm3/mol, and Tb
=
450.38 K
Using Eq. (13-18) for vapor pressure:
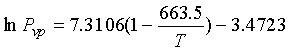
13.4
For pure caffeine, Tb![]() 640
K, Tc = 872 K, Vc = 488.5 cm3/mol,
and Pc = 41.46 bar
640
K, Tc = 872 K, Vc = 488.5 cm3/mol,
and Pc = 41.46 bar
Using the PR EOS for density estimate for 5 mol % caffeine
CO2 mixture at 80 bar, 310 K ![]() =
1151 kg/m3. The vapor pressure of liquid caffeine is approximated
by Eq. (13-18)
=
1151 kg/m3. The vapor pressure of liquid caffeine is approximated
by Eq. (13-18)
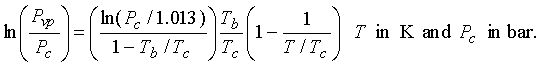
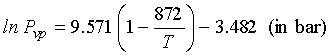
14.2
Using the PR EOS,
![]()
![]()
![]()
![]()
14.6
(a) ![]()
(b) ![]()
(c) Using the RKEOS and a suitable mixing rule,
amix
and bmix parameters can be calculated and used to calculate
all PVTN properties. With an ideal-gas state
Cp
all derived properties needed for the cycle calculation can be obtained
using a departure function approach. Then,
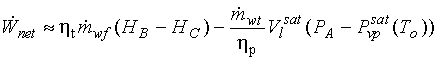
14.8
(a) 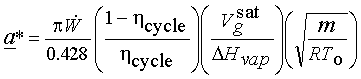
(b) ![]() (about 150 times smaller!)
(about 150 times smaller!)
14.12
(a) and (b) ![]()
so CTI’s claim of 1 kWhr
production violates the 2nd Law limit
(c) All heat transfer and work production and utilization
steps have some irreversibility in practical systems.
14.14
(a) ![]()
(b) no fundamental laws or concepts are violated
(c) ![]() about 17% more area for heat exchange is required for the GH process
about 17% more area for heat exchange is required for the GH process
(d) ![]()
NB: If an electric motor drive is used for the feed pump in the conventional Rankine system, then the net output would be reduced possibly accounting for the discrepancy.
15.1
![]()
15.3
x (Naphthalene) = 0.196
15.6
(c) q (critical quality) = ![]()
(e) O2: q = 0.54 and H2: q = 0.36
15.12
xKCl = 0.413 and
T
= 624 K
15.13
![]()
15.15
Anesthetic pressure of CCl4-![]() 4.9
4.9![]() 10-3 bar.
10-3 bar.
15.19
(a) ![]()
(b) ![]()
(c)![]()
(i) ![]() ,
solution is regular
,
solution is regular
(ii)![]() ,
less structural ordering in mixture versus pure state
,
less structural ordering in mixture versus pure state
(iii)![]() ,
more structural ordering in mixture versus pure state, implies
,
more structural ordering in mixture versus pure state, implies ![]()
16.9
(a) yA = 0.764 yB
=
0.236
(b) not in equilibrium, estimate yB assuming
equilibrium
16.12
![]()
16.14
![]()
16.16
![]() using
expansion valve
using
expansion valve
![]() using
expansion turbine
using
expansion turbine
Consider net energy, entropy, and work flows using 1st
and 2nd Law concepts to show process is not feasible as described.
16.20
(a) P = 21.9 bar
(b) T = 51.5 K
(c) ![]()
16.22
T, K Cp
(effective),
J/gK
293
4.69
313
7.20
333
8.29
353
6.36
373
3.64
17.3
(a) ![]() ;
at A: L1-L2-H-G and at
B:
L1-H-I-G
;
at A: L1-L2-H-G and at
B:
L1-H-I-G
(b) L1 = 0 and M1
= 0 use P-explicit EOS to determine required Aijk
and
Aij
derivatives
(c) ![]()
(d) ![]()
![]() in this case
in this case
17.6
d ln P/d(1/T) = ![]()
18.1
![]()
18.4
![]() J/kg
mol
J/kg
mol
18.5
Ti = 269 K = -4oC
(winter!)
18.6
Tbath = 317 K,
efficiency = 0.065
18.8
z = depth = 764 m
18.9
![]() (tube
bottom) = 0.501, if L
(tube
bottom) = 0.501, if L ![]() 4/7
4/7
18.11
![]()
18.14
ytritium = 0.065 and ydeuterium
= 0.538 at rim
18.16
h = 252 m, for equilibrium ocean, fresh water
cannot rise above a level 252 m below sea surface. For the well-mixed case,
process becomes feasible if Z = depth > 9850 m (32,000 ft.).
19.1
![]()
19.2
![]()
19.5
![]()
19.7
xbutanol = 0.436
19.8
(a) 0.046 J/m2
(b) 296 K
| Home | Webmaster |