
The ability to precisely control parameters such as substrate, flow rate, buffer composition, and surface chemistry in these microscale devices makes them ideal for a broad spectrum of cell biology-based applications, ranging from the high-throughput screening (HTS) of single cells to three-dimensional scaffolds for tissue and organ culture. Microscale devices offer the possibility of solving system integration issues for cell biology, while minimizing the necessity for external control hardware. Many applications, such as enzymatic library screening in bacteria, are currently carried out as a series of multiple, labor-intensive steps required in the assay process, from growing single colony isolates to plate-based assays and recovery. While the industrial approach to complexity has been to develop elaborate mechanical HTS workstations, this technology comes at a price, requiring considerable expense, space and labor in the form of operator training and maintenance. For small laboratories or research institutions, this technology is simply out of reach. Devices consisting of addressable microscale fluidic networks can dramatically simplify the screening process, providing an environment where a bulk cultures can be injected and compartmentalized into sub-nanoliter aliquots for sensitive single-cell assays.
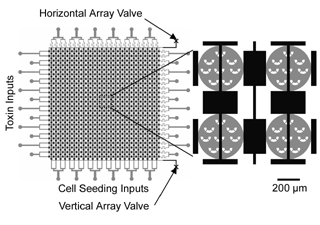
While microfluidics-based technologies are typically championed for aspects such as reduced reagent consumption and disposability, the volume reduction from milliliters in bulk culture systems to sub-microliters in microfluidic devices presents several challenges for cell culture, including adequate media exchange rates and the ability to control device seeding density. In recent work reported in Lab on a Chip (Ref 1), we described the development of a microsieve-based platform for precisely trap and seed defined numbers of cells in microchambers for cell cytotoxicity measurements. Computational fluid dynamics was utilized as an essential tool to define the cell sieve geometries and sieve distribution within the microchambers, enabling the end user to uniformly trap a defined number of cells/sieve using pressure based flow.
   |
Present Post-Doctoral Fellows
 |
Dr. Zhanhui Wang |
 |
Dr. Min-Cheol Kim |
Present Graduate Students
 |
Raymond Lam (Doctoral Student) |
Present Undergraduate Students
Visitors
Alumni
Collaborators
Dr. Manuel Marquez-Sanchez, Philip Morris USA
Sponsors
INEST Research Grant, Philip Morris USA
Links
Interdisciplinary Network of Emerging Science and Technologies (INEST)
Refereed Publications and Conference Proceedings
1. Z. Wang, M.-C. Kim, M. Marquez, and T. Thorsen. High-Density Microfluidic Arrays for Cell Cytotoxicity Analysis. Lab Chip 7: 740-745 (2007) (PDF)
2. Z.Wang, M.-C. Kim, M. Marquez and T. Thorsen. A Microfluidic Array with Micro Cell Sieves for Cell Cytotoxicity Screening. Materials Research Society 2007 Spring Meeting. San Francisco, CA. April 11, 2007.
3. M.-C. Kim, Z.H. Wang, R.H.W. Lam and T. Thorsen. Simulation of biological cell transport in microfluidic devices. Nanotech 2007. Santa Clara, CA. May 23, 2007.
4. Z.H. Wang, M.-C. Kim, M. Marquez and T. Thorsen. A Microfluidic Array with Micro Cell Sieves for Cell Cytotoxicity Screening. Nanotech 2007. Santa Clara, CA. May 24, 2007.