
Polymeric microspheres have received much attention as controlled drug delivery systems. Several methods are used for their production, including the widely-used solvent evaporation method. The solvent evaporation method is preferable to other methods such as spray drying, homogenization, and sonication as it only requires ambient temperature conditions and mild emulsification techniques. Although the method is conceptually simple, with the microspheres formed as an emulsion of the polymer/drug mixture, many variables affect the size and composition of the final product, including the polymer/drug ratio, phase ratio of the emulsion system, drug solubility, organic solvent composition, emulsion concentration, and apparatus design.
Conventional solvent evaporation protocols for microsphere manufacture use a stirring process to shear the polymer/drug mixture into the continuous phase, generating product with significant polydispersity. Eliminating this polydispersity is clearly desirable, as particles with uniform surface/volume ratios will provide a source of uniform drug release within a biological system. Using pressurized microfluidic crossflow devices, we have generated monodisperse polymeric microspheres with tunable diameters ranging from 1 - 50 µm. These devices combine crossflow and viscoelastic shear to generate a constant stream of droplets. Unlike previously described crossflow techniques for generating water-in-oil (W/O) emulsions, in which the discontinuous phase is forced through narrow pores or capillaries into an open continuous phase, we generated micrspheres in a silicone-based microfluidic device at the junction of two microfluidic channels containing solubilized biodegradable polymer (Eudragit) and an oil-surfactant mixture, respectively. The water partially obstructs the flow at the junction, but is not ruptured at the channel interface as in traditional crossflow devices. Microsphere formation is achieved by high shear forces generated at the leading edge of the water perpendicular to the oil flow. The microsphere size and frequency can be precisely controlled by modifying the relative pressure of the polymer/oil solutions, the microchannel geometry, and the relative solubility of the dispersion agent in the continuous phase.
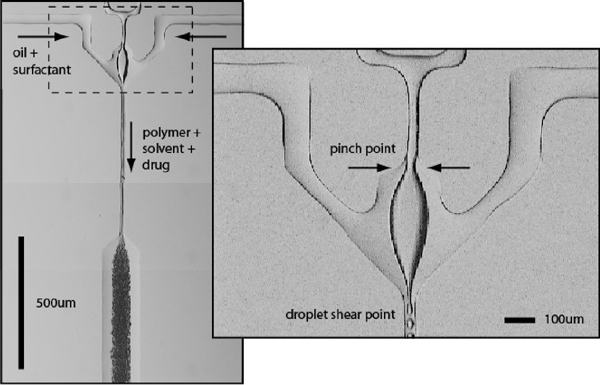 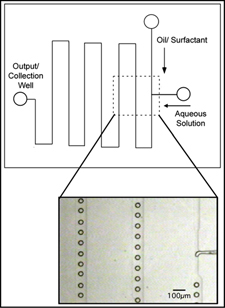  |
While bulk microparticle manufacture using the solvent evaporation method requires constant stirring for up to 24 hours to remove the solvent from the formed particles, we predict that the microspheres will rapidly lose solvent to the continuous oil phase. This hypothesis is supported by collaborative work that we have done in which micron-scale latex colloidal assemblies were generated in polyurethane and silicone microfluidic devices. As the encapsulated colloidal mixture flowed down the microchannels, water was continuously lost to the oil phase. Water was slightly soluble in the test oils (mineral oil and fluorinated silicone), driving the transfer of water out of the droplets. The resultant particles (~10 µm average diameter) were stable and resistant to coalescence in bulk. Final particle size was a product of the original droplet size, solvent composition and solvent/solute ratio.

Microgels are promising materials in drug delivery and biomedicine. Although monodisperse microgels would offer considerable advantages, most microgels investigated and used today are polydisperse in size. We have also fabricated 10 um sized monodisperse microgels by emulsifying an aqueous dextranhydroxyethyl methacrylate (dex-HEMA) phase within an oil phase at the junction of microfluidic channels. Dex-HEMA microgels are biodegradable and are ideally suited for the controlled delivery of proteins.
Microfluidic Electrospinning of Nanofibers
Advances in the fields of electronics, optics, and medicine have motivated the production of a variety of nano-dimensional functional materials ranging from thin films to fibers. The versatile technology of electrospinning for the preparation of polymer nanofibers has been known since early 1930s. In the conventional electrospinning method, a syringe with a fixed diameter of 0.3-1 mm has been used as an electrospinning source. The pendant polymeric droplet exiting the tip of the needle, when subjected to strong electric field, will deform into a Taylor cone and form a liquid jet. The jet undergoes an electrically-induced bending instability which results in strong looping and the stretching of the jet. After the solvent evaporation, ultra-thin fibers are deposited on the counter electrode. We have been working on the development of devices that replace the conventional needle-based manifold with microfluidic channels cast in (poly) dimethylsiloxane. Microfluidic electrospinning devices have several advantages over conventional manifolds, including rapid prototyping, disposability, and the ability to electrospin single and multi-component fibers in parallel.
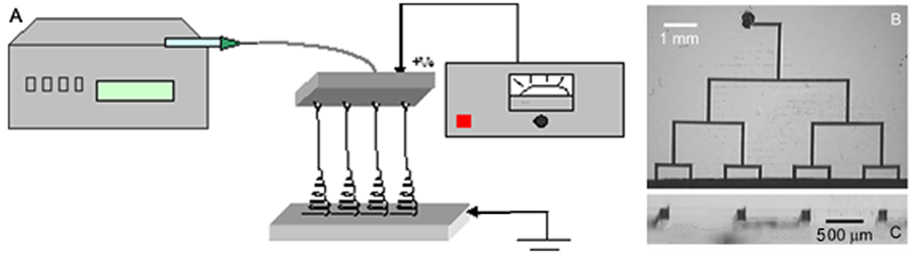 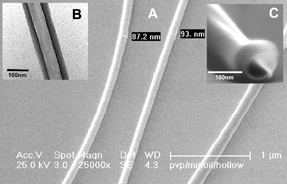 |
Present Post-Doctoral Fellows
 |
Dr. Yasmin Srivastava |
Present Graduate Students
 |
J.P. Urbanski (Doctoral Student) |
Present Undergraduate Students
Visitors
Alumni
Collaborators
David Pine, Dept. of Physics, N.Y.U.
Jo Demeester, Ghent University, Belgium
Dr. Manuel Marquez-Sanchez, Philip Morris USA
Ignacio Loscertales, Malaga University, Spain
Links
Interdisciplinary Network of Emerging Science and Technologies (INEST)
Support
INEST Research Grant, Philip Morris USA (Nanofiber synthesis)
National Science and Engineering Research Council of Canada (PGSM Scholarship).
Refereed Publications and Conference Proceedings
1. T. Thorsen, R.W. Roberts, F.H. Arnold, S.R. Quake. Dynamic pattern formation in a vesicle-generating microfluidic device. Phys. Rev. Lett . 86 (18): 4163-4166 (2001)
2. G.-R. Yi, T. Thorsen, V.N. Manoharan, M.-J. Hwang, D.J. Pine, S.R. Quake, S.-M. Yang. Generation of Uniform Colloidal Assemblies in Soft-Microfluidic Devices. Advanced Materials 15 (15): 1300-1304 (2003) (PDF)
3. G.-R.Yi, S.-J. Jeon, T. Thorsen, V.N. Manoharan, D.J. Pine, S.R. Quake, S.-M. Yang. Generation of Uniform Photonic Balls by Template-Assisted Colloidal Crystallization" Synthetic Metals 139 (3): 803-806 (2003) (PDF)
4. B.G. De Geest, J.P. Urbanski, T. Thorsen, S.C. De Smedt, and J. Demeester. Monodisperse microgel synthesis inside microfluidic devices. Langmuir 21(23): 10275-10279 (2005) (PDF)
5. I.G. Loscertales, Juan E. Díaz Gómez, M. Lallave, J. M. Rosas, J. Bedia, J.Rodríguez-Mirasol, T. Cordero, M. Marquez, S. Shenoy, G. E. Wnek, T. Thorsen,A. Fernández-Nieves and A. Barrero. Coaxial electrospinning for nanostructured advanced materials. Materials Research Society Fall Meeting, Boston, MA, November 27, 2006.
6. Y.N. Srivastava, M. Marquez and T. Thorsen. Microfluidic Electrospinning of Hollow and Core/Sheath Nanofibers. Materials Research Society 2007 Spring Meeting. San Francisco, CA. April 12, 2007.
7. Y. Srivastava, I. Locertales, M. Marquez and T. Thorsen. Electrospinning of Hollow and Core/Sheath Nanofibers Using Microfluidic Manifolds. Microfluidics Nanofluidics In Press (2007) (PDF)
8. Y. Srivastava, M. Marquez and T. Thorsen. Multi-jet electrospinning of conducting nanofibers from microfluidic manifolds. J. Appl. Polymer Sci. 106: 3171-3178 (2007) (PDF)