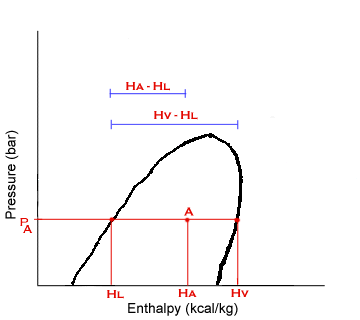

At Point A:
x = vapor fraction
1-x = liquid fraction
Point A represents a mixture of saturated liquid and vapor at pressure, PA. The enthapy at A, HA, is a weighted average of the enthalpies of the saturated phases:
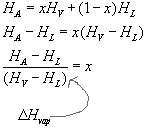
This is the same formula used to balance a seesaw.
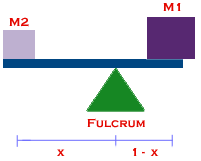
Hence the name "lever rule." The lever rule can also be used for other properties besides H, like volume.
VA = xVV + (1-x)VL
VA = (0.3)(43400) + (1-0.3)(1.003)
Notice that the saturated liquid's contribution to the volume is
almost negligible compared to the contribution of the vapor.