Problem 3 (Due Wednesday, September 15)
Chemical Vapor Deposition (CVD) is a method for growing thin film coatings. This process is extensively used by the microelectronics industry to fabricate integrated circuits, such as microprocessors. The films, typically a micron or less in thickness, are grown on silicon wafers, which are several hundred millimeters in diameter and are a few millimeters in thickness.
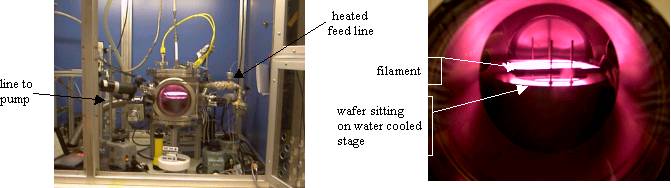
Photographs of one of the CVD reactor in my laboratory is shown below. Some of the reactor parts have been labeled. In the process, molecules enter a vacuum chamber in gaseous form. The pressure of the chamber is controlled by using a vacuum pump and a valve. Subsequent chemical reactions, driven either by thermal or electrical energy, produce film deposition on the silicon wafer substrate. The temperature of the substrate is maintained at 25 °C by backside water cooling.

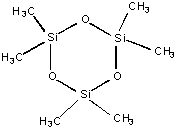
My research group is investigating how the choice of reactant molecule determines the composition and properties of the deposited films. One of the molecules under study is D4. The following information was available from the catalog from which the D4 was purchased:
| Melting point: 17 °C
Boiling point: 175 °C Vapor Pressure @ 23 °C : 1 torr 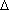 Hvap:
10.9 kcal/mol Hvap:
10.9 kcal/mol
Molecular Weight: 296.61 |
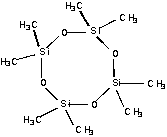 |
b) If the gas inside the reactor is pure D4, what is the maximum operating pressure which avoids condensation of liquid D4 on to the 23 °C substrate?
c) In order for reactant to flow into the CVD chamber, heating tape is used to elevate the temperature of the crucible which contains a supply of the pure reactant. The higher temperature increases the vapor pressure in the crucible and the resultant pressure difference allows the reactant to flow into the CVD chamber. If a vapor pressure of 10 torr is required, to what temperature must the crucible be heated?
d) Sketch a P-T diagram and show the location of the three conditions defined in parts a, b, and c of this problem.
e) A related molecule, D3, is also under investigation. What phase or phases of D3 exist at equilibrium under ambient conditions.
| Melting point: 65 °C
Boiling point: 134 °C Vapor Pressure @ 70 °C : 70 torr 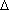 Hvap:
9.5 kcal/mol Hvap:
9.5 kcal/mol
Molecular Weight: 222.46 |
 |