10.213 Fall 1999
Problem 4 (Due Friday, September 17)
For methane, the pressure explicit virial
equation of state (EOS) truncated to four terms is:
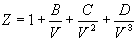
At 0 °C, the values of the virial coefficients
are:
B= -53.4 cm3/mol
C= 2,620 cm6/mol-2
D= 5000 cm9/mol-3
-
Graph the compressibility, Z, of methane at
0 °C, as a function of pressure from zero to 150 bar.
-
To what pressure is the ideal gas law a good
approximation?
-
To what pressure is the volume explicit virial
EOS truncated to one term, Z=1+BP/RT, a good approximation?
![]()