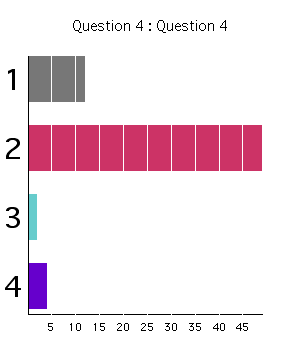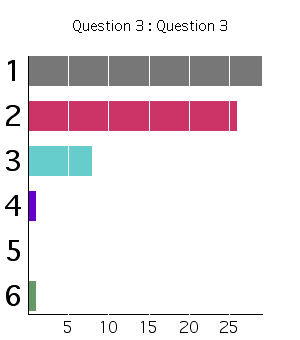
Chapter 7 Question 9 Answer:
(2) T1 = T2
From the First Law, Du = q - w. q=0 since the container is thermally-insulated. w=0 since the container is rigid (or if you draw your system around the gas, because the external pressure = 0). Therefore, Du=0. So for an ideal gas then, the temperature is constant since du=cvdT.
Class Response (2003):
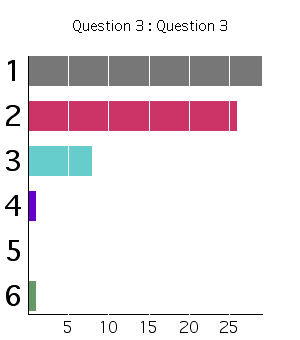
Class Response (2002):