|
- Work done in any adiabatic (
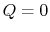 ) process is a function of state.
We can write the first law, setting the heat transfer
term equal to zero, as
) process is a function of state.
We can write the first law, setting the heat transfer
term equal to zero, as
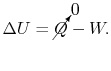 |
(2..3) |
Since 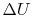 depends only on the state change, now
depends only on the state change, now 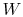 can be
found as a function of the state change.
can be
found as a function of the state change.
Figure 2.3:
The change in energy between
two states is not path dependent.
|
|
- For a cyclic process heat and work transfers are numerically equal
Figure 2.4:
Since energy is a
function of state only, any process that returns a system to its
original state leaves its energy unchanged.
|
|
therefore
and
Douglas Quattrochi
2006-08-06
|

