|

| |
Genetic Dissection of Molecular Pathways Implicated in Autism
 Defining molecular pathways that are
dysfunctional in autistic spectrum disorders (ASDs) is key to understanding
their pathogenesis. In Alzheimerís and Parkinsonís Disease, identification of
single gene mutations in the 5-10% of genetic cases have revealed core molecular
pathways that are altered, including in the larger category of non-genetic
cases. A key question is whether a similar molecular pathway will emerge for
autism based on the recent identification of defined mutations and de novo
genome copy number variations that account for 10-20% of ASDs. Current evidence
suggests the disease may result from disruption in synapse formation and
synaptic plasticity during development, with several autism-linked mutations in
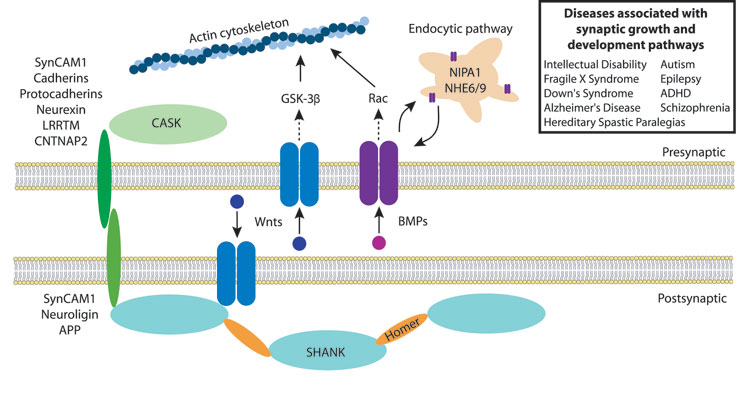
humans mapping to the endosomal pathway. Recent work from our laboratory has
indicated that regulation of presynaptic endosomal trafficking plays a key role
in synaptic growth regulation by controlling the activity of retrograde synaptic
growth signals processed by presynaptic growth receptors. Protein complexes
regulating signal transduction are directed to specific subcellular membrane
domains along their journey through the endocytic pathway. These temporally and
spatially regulated trafficking steps result in distinct signaling properties at
various points along this route, due to compartment-specific post-translational
modifications and degradative events, or interactions with local binding
partners. In neurons, growth factor signaling controls the expansion of synaptic
arbors in response to activity and external stimuli, leading to long-lasting
changes in synaptic strength and connectivity that underlies learning and
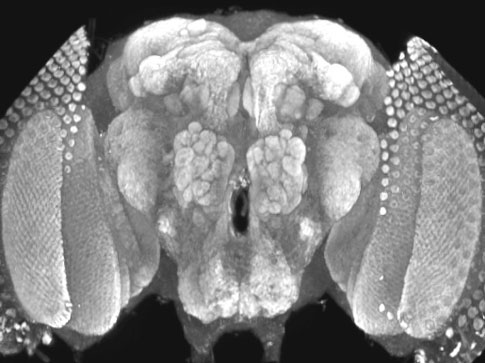
memory. The Drosophila larval neuromuscular junction (NMJ) serves as a
useful model for synaptic growth, as the muscle surface area expands 100-fold
over 4 days of larval development, requiring increased input from its
innervating motor neuron to drive contraction. NMJ synaptic arbors expand by
adding matched pre- and post-synaptic specializations (termed synaptic boutons),
in response to motor neuron synaptic activity, retrograde signals from the
muscle to the neuron, and anterograde signals from the neuron to the muscle.
Defining the mechanisms by which protein traffic between endosomal intermediates
at synapses controls the output of signal transduction pathways leading to
synaptic growth is a key area of study. We are using Drosophila to
explore the mechanisms by which the newly identified autism-associated endosomal
protein, NHE9, couples alterations in neuronal activity to modifications of
synaptic connectivity. NHE9 is one of several newly identified genetic links
that indicate abnormal endosomal trafficking may lead to autism. Defining the
role of NHE9 in endosomal trafficking at synapses will allow us to place this
important protein into the emerging picture of a dysfunctional molecular cascade
that regulates synaptic plasticity and development that may underlie autism. Defining molecular pathways that are
dysfunctional in autistic spectrum disorders (ASDs) is key to understanding
their pathogenesis. In Alzheimerís and Parkinsonís Disease, identification of
single gene mutations in the 5-10% of genetic cases have revealed core molecular
pathways that are altered, including in the larger category of non-genetic
cases. A key question is whether a similar molecular pathway will emerge for
autism based on the recent identification of defined mutations and de novo
genome copy number variations that account for 10-20% of ASDs. Current evidence
suggests the disease may result from disruption in synapse formation and
synaptic plasticity during development, with several autism-linked mutations in
humans mapping to the endosomal pathway. Recent work from our laboratory has
indicated that regulation of presynaptic endosomal trafficking plays a key role
in synaptic growth regulation by controlling the activity of retrograde synaptic
growth signals processed by presynaptic growth receptors. Protein complexes
regulating signal transduction are directed to specific subcellular membrane
domains along their journey through the endocytic pathway. These temporally and
spatially regulated trafficking steps result in distinct signaling properties at
various points along this route, due to compartment-specific post-translational
modifications and degradative events, or interactions with local binding
partners. In neurons, growth factor signaling controls the expansion of synaptic
arbors in response to activity and external stimuli, leading to long-lasting
changes in synaptic strength and connectivity that underlies learning and
memory. The Drosophila larval neuromuscular junction (NMJ) serves as a
useful model for synaptic growth, as the muscle surface area expands 100-fold
over 4 days of larval development, requiring increased input from its
innervating motor neuron to drive contraction. NMJ synaptic arbors expand by
adding matched pre- and post-synaptic specializations (termed synaptic boutons),
in response to motor neuron synaptic activity, retrograde signals from the
muscle to the neuron, and anterograde signals from the neuron to the muscle.
Defining the mechanisms by which protein traffic between endosomal intermediates
at synapses controls the output of signal transduction pathways leading to
synaptic growth is a key area of study. We are using Drosophila to
explore the mechanisms by which the newly identified autism-associated endosomal
protein, NHE9, couples alterations in neuronal activity to modifications of
synaptic connectivity. NHE9 is one of several newly identified genetic links
that indicate abnormal endosomal trafficking may lead to autism. Defining the
role of NHE9 in endosomal trafficking at synapses will allow us to place this
important protein into the emerging picture of a dysfunctional molecular cascade
that regulates synaptic plasticity and development that may underlie autism.
|