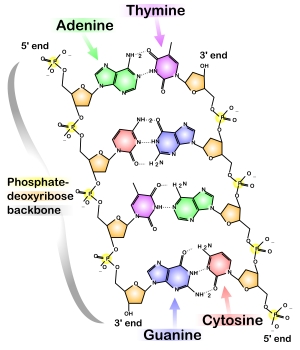
The final aspect of DNA that we will examine is the electrostatic energy due to DNA's overall negative charge. The figure below shows that each sugar-base unit of DNA contains one phosphate with an overall charge of -e.

[Negative phosphates attached to sugar-base units of DNA. Sugars and phosphates make up the backbone of DNA. Image courtesy of Wikipedia.]
As there are two such units per base pair, there are thus 2 negative charges in the space of 0.34 nm, and we can consider double-stranded DNA as a single-stranded polymer with negative charges located every b = 0.17 nm.
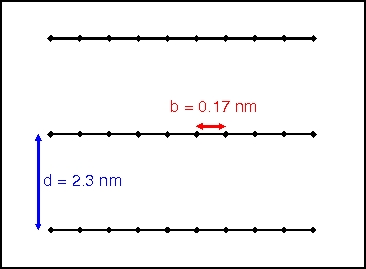
[Schematic representation of DNA strands stacked on top of each other when coiled in capsid.]
If the DNA were in a vacuum, the calculation of electrostatic energy would be straightforward -- using Coulomb's law, we could determine that the electrostatic energy is simply a sum of e2/r for all of the charges interacting with one another. However, DNA in a capsid is immersed in a solution of water and various ions derived from common biological salts (Na+, Ca2+, etc.).
Since water is a polar fluid, it acts as a dielectric material that mitigates the strength of the electric field from each point charge. This could be accomodated in the electrostatic potential by dividing it by a dielectric constant. The ions in solution create a more complicated picture. The positive ions will tend to cluster around the negative charges of DNA, thus further shielding the charge. To determine exactly how this will impact the electrostatic potential, we need to first consider modifying Poisson's equation.
In the simple scenario of a charge in a dielectric medium, we use the Poisson equation:
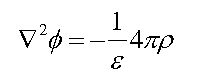
With ions in solution, however, we must use the Poisson-Boltzmann equation given below:
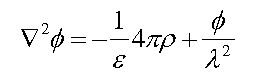
In the interests of time (and not frying one's brain), the solution to this equation for a point charge is:
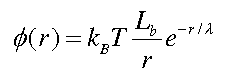
Of note in this equation is Lb, the Bjerrum length of water (0.71 nm). Lb is the characteristic length at which point the energy of electrostatic interactions is of the order of thermal fluctuations. Also, as we expected, the salt ions have screened the charge -- this is seen with the addition of the exponential factor (with screening length lambda) to the potential function. This exponential decrease in the potential is shown in the graph below, along with the standard 1/r potential.
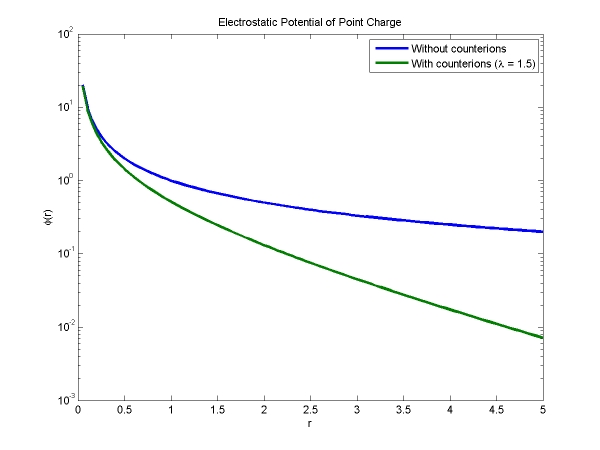
Given that the strands are separated by d = 2.3 nm and the screening length is ~1 nm, we already know the potential for one charge interacting with another at a distance d, but we need to figure out an estimate for the total number of charges interacting with that one charge. If we consider all of the charges on neighboring strands within a distance (21/2)d of our "test" charge, then we find that there are ~30 such charges on each of the two nearest strands to our test charge. We could put in the distance r to each one of these charges, but since we are neglecting all the charges beyond a certain distance, it is reasonable to assume that r = d = 2.3 nm for all of the charges.
Finally, we know that there are a total of L/b charges for the entire DNA strand, so the total electrostatic energy should be E = 30 * (L/b) * (Lb/d)* exp(-r/lambda) kBT. (Note that the fact that there are two neighboring strands (one in either direction) doesn't seem to show up -- that is because we have multiplied by 1/2 to avoid double counting interactions. Since this is an order of magnitude estimate, however, factors of 2 should be insignificant.) Plugging in actual numbers, we find that the energy cost due to electrostatic interactions is Ees = 30 * (6800/0.17) * (0.71/2.3) * exp(-2.3) kBT ~ 4 * 104 kBT.
The energy cost due to electrostatic interaction between the negative charges of DNA is larger than the energy cost of entropy reduction or bending by at least two and one orders of magnitude respectively. In fact, experiments show that ~105 kBT is required to pack the DNA of Phi29 into its capsid, so this estimate is not terribly far off from reality.
To put this kind of energy into another biological perspective, the hydrolysis of one ATP molecule into ADP yields ~10 kBT of energy. Therefore ~104 ATP molecules' worth of energy are required to pack the DNA into a capsid. Put another way, there are 2 * 104 base pairs of DNA in Phi29's genome, so it is necessary to use 1 ATP molecule (or its energy equivalent) to pack every 2 base pairs of DNA (for just a single copy of the phage!).