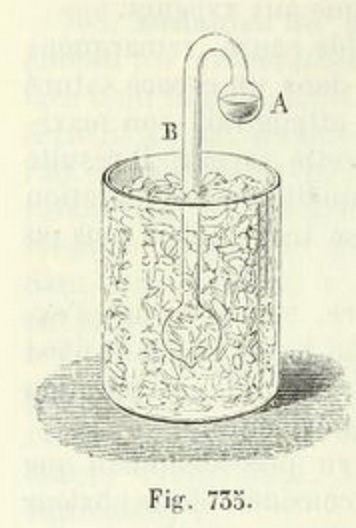
From Physique by Ganot & Maneuvrier [Paris: Hachette, 1887]
The ancients regarded heat and cold as opposing principles with equal standing. In the well-known scheme of the four elements, heat was considered the mean of fire and air, cold that of water and earth. This idea persisted into early modern physics, with heat and cold often regarded as two distinct, invisible fluids with opposite "thermal charge" pouring in and out of bodies. Readers of this blog may recall, from the article on the solidification of mercury, that many Eighteenth Century thermometers showed the Delisle scale, in which water boils at 0°D and freezes at +150°D.
Thermodynamics was perhaps the quintessentially Nineteenth Century branch of physics : born from the steam engine, headed inexorably toward the atomic realm. The thermodynamicists' identification of heat with energy left no room for cold as anything but an absence, however much the use of "coldness" (1/T) instead of temperature would have mathematically simplified the formalism. With the advent of statistical mechanics, it became almost self-evident that heat is a measure of microscopic agitation, while cold is a word almost without meaning.
All this on the theoretical front --- but at the same time the new experimental science eventually named cryogenics was developing apace, and ever-lower temperatures were being attained in the "quest for absolute zero". (Had "coldness" triumphed over "temperature", this would have been "the quest for infinity", and its impossible nature would have been obvious.) Among the early pioneers of cryogenics (and indeed much of experimental physical-chemistry) was this week's featured author, the physician and chemical engineer William Hyde Wollaston (1766-1828). The "cryophorus" was Wollaston's main contribution to low-temperature research. This device consists of two interconnected glass bulbs (one of them containing water) from which all the air has (ideally) been removed. If the dry bulb is rapidly cooled, the water in the other will distill so swiftly that evaporative cooling freezes the liquid. While not a practical method of producing ice, the cryophorus inspired Faraday and others to attain lower temperatures and even to liquify some common gases. Although its operation was well understood in terms of early Nineteenth Century thermal theory, the cryophorus seemed counterintuitive at a less abstract level : it transmitted cold. Reading the paper, one notes the tension between Wollaston's intellectual belief that heat is primary and his visceral feeling that he is exploring the depths of cold.

W. H. Wollaston by Sir Thomas Laurence. [National Library of Medicine]
ON A METHOD OF FREEZING AT A DISTANCE
by Wm. Hyde Wollaston, M.D., Secretary of the Royal Society
[Phil. Trans. R. S. L. 103, 71 (1812 December 17)]
Wollaston's words are in bold.
That a fluid, from which a portion is evaporated, becomes colder in consequence of the heat absorbed by that part which assumes the gaseous state: that fluids rise in the state of vapour at a lower temperature when the pressure of the atmosphere is removed, and consequently may be cooled to a lower degree by evaporation in vacuo than in the open air, are facts too well known to need confirmation before the Members of this Society by any new experiments.
Nevertheless, a new mode of applying the most established principles may deserve to be recorded, if it assist the illustration of them, and be instructive from the novelty of the view in which it exhibits a certain class of phenomena ; although no immediate use be at present proposed, to which it can be applied with advantage.

Aristocratic physics-student Catherine Molchanova with a typical Eighteenth Century vacuum pump. [Painting by D. G. Levitzky, 1776]
If an attempt were made to freeze water by evaporation, without other means than the vacuum of an air-pump, the pump must be of the best construction, and though the quantity of water be small, the receiver must be of large dimensions, otherwise its capacity would set too confined a limit to the quantity of vapour that will rise, and consequently to the degree of cold produced.
Supposing the commonly received estimates to be correct, as to the quantities of heat, that become latent in the conversion of ice into water, and of water into steam, being 140° and 960° respectively ...
[The modern reader will note that in 1812 "heat" and "temperature" were not yet distinct concepts. Wollaston's temperatures are in degrees Fahrenheit.]
Supposing the commonly received estimates to be correct, as to the quantities of heat, that become latent in the conversion of ice into water, and of water into steam, being 140° and 960° respectively, we should find the following statement to be not far from the truth.
If 32 grains of water were taken at the temperature of 62°, and if one grain of this were converted into vapour by absorbing ing 960°; then the whole quantity would lose (960°/32) = 30°, and thus be reduced to the temperature of 32°.
If from the 31 grains, which still remain in the state of water, 4 grains more were converted into vapour by absorbing 960°; then the remaining 27 grains must have lost (4/27) of 960° = 142°, which is rather more than sufficient to convert the whole into ice. In an experiment, conducted upon a small scale, the porportional [sic] quantity evaporated did not much differ from this estimate.
If it be also true, that water in assuming the gaseous state, even at a low temperature, expands to 1800 times its former bulk ; then in attempting to freeze the small quantity of water abovementioned, it would be requisite to have a dry vacuum with the capacity of 5 × 1800, or equal to that of 9000 grains of water.

Apparatus used by John Leslie to manufacture ice. The tripods supporting the water dishes of Figures 9 and 10 are standing in pools of sulphuric acid.
As a means of avoiding the necessity of so large a vacuum, Mr. Leslie had recourse to the ingenious expedient of employing an extensive surface of sulphuric acid, for the purpose of absorbing the vapour generated in the course of the experiment, and by that means contrived to freeze much larger quantities of water, than could otherwise have been done, and by a far less laborious process.
But even in this method the labour is not inconsiderable, and the apparatus, though admirably adapted to the purpose for which it is designed, is large and costly. I have therefore thought the little instrument I am about to describe may possess some interest, as affording a readier and more simple mode of exhibiting so amusing and instructive an experiment.
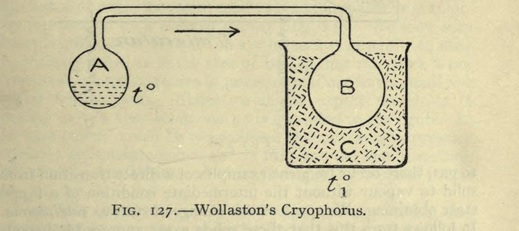
Let a glass tube be taken, having its internal diameter about (1/8) of an inch, with a ball at each extremity of about one inch diameter ; and let the tube be bent to a right angle at the distance of half an inch from each ball. One of these balls should contain a little water --- if the ball be more than half full, it will be liable to burst by the expansion of water in freezing --- and the remaining cavity should be as perfect a vacuum as can readily be obtained. The mode of effecting this is well known to those who are accustomed to blow glass.
One of the balls is made to terminate in a capillary tube, and when water admitted into the other has been boiled over a lamp for a considerable time, till all the air is expelled, the capillary extremity, through which the steam is still issuing with violence, is held in the flame of the lamp till the force of the vapour is so far reduced, that the heat of the flame has power to seal it hermetically.
When an instrument of this description has been successfully exhausted, if the ball that is empty be immersed in a freezing mixture of salt and snow, the water in the other ball, though at the distance of two or three feet, will be frozen solid in the course of a very few minutes.
The vapour contained in the empty ball is condensed by the common operation of cold, and the vacuum produced by this condensation gives opportunity for a fresh quantity to arise from the opposite ball, with proportional reduction of its temperature.
According to a theory that does not admit of positive cold, we should represent the heat of the warmer ball to be the agent in this experiment, generating steam as long as there remains any excess of heat to be conveyed. But if we would express the cause of its abstraction, we must say that the cold mixture is the agent, and may observe, in this instance, that its power of freezing is transferred to a distance, by what may be called the negative operation of steam.
The instrument, by which this is effected, may aptly be called a Cryophorus, which correctly expresses its office of frost-bearer.
From A Student's Heat by Ivor Blashka Hart [London: Dent, 1916]
The cryophorus was well-known all through the Long Nineteenth Century, but mainly as an immensely popular lecture-demonstration for first-year physics students. Many inventors (among them Nikola Tesla in the early 1900s) were fascinated by the device and imagined dramatic applications, but these came to nothing, although "freezing rays" became a staple of pulp science-fiction. While the practical use of evaporative cooling (already known before Wollaston's use of it in the low-temperature field) remained confined largely to refrigeration and air-conditioning -- the importance of which for modern civilisation, however, can hardly be overstated -- its scientific value became most evident in the 1990s, when magnetic evaporative-cooling techniques made possible the creation of Bose-Einstein condensates.