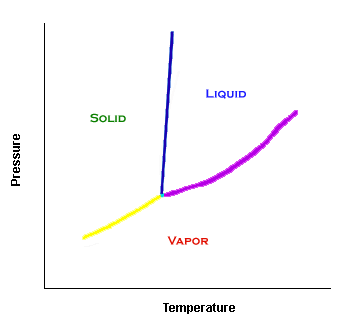
 The three
phases of a pure substance are solid, liquid, and vapor. Vapor
is the bottom area, below the yellow and purple lines. You can rationalize this: At very
low pressures, a substance will be in the vapor phase.
The three
phases of a pure substance are solid, liquid, and vapor. Vapor
is the bottom area, below the yellow and purple lines. You can rationalize this: At very
low pressures, a substance will be in the vapor phase.3PHASES
 The three
phases of a pure substance are solid, liquid, and vapor. Vapor
is the bottom area, below the yellow and purple lines. You can rationalize this: At very
low pressures, a substance will be in the vapor phase.
The three
phases of a pure substance are solid, liquid, and vapor. Vapor
is the bottom area, below the yellow and purple lines. You can rationalize this: At very
low pressures, a substance will be in the vapor phase.
The solid phase occurs in the region to the left of the almost-vertical line (shown in blue). This makes sense: if something is at low temperature and high pressure, chances are that the substance is solid.
The remaining region is the liquid phase. It is located to the right of the blue line.
Phase Equilibrium Lines
The lines between different phases signify a set of points where the phases are in equilibrium. The sublimation curve (yellow) shows when both solid and vapor can exist. The fusion curve (blue) represents when both solid and liquid are in equilibrium. If the fusion curve points toward the left (like in a water phase diagram), the liquid phase is denser than the solid phase. The vaporiation curve (purple) is when vapor and liquid are in equilibrium.
The three lines meet at the triple point, a specific temperature and pressure where all three phases are in equilibrium.
The vaporization curve ends at the critical point. 10.213 calculations usually do not involve temperatures higher than the critical temperature.