POINTS
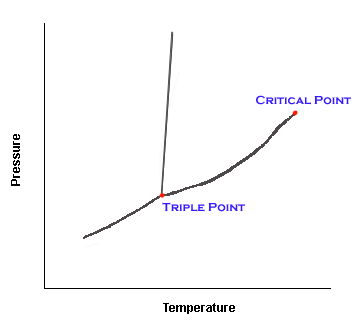 The three phase equilibrium curves meet at the triple
point. At the triple point, all three phases (solid, liquid, and gas) are in
equilibrium. Since the triple point is a point, there is only one temperature and one
pressure where the three phases will exist. This fact often helps in identifying compounds
or in problem solving.
The three phase equilibrium curves meet at the triple
point. At the triple point, all three phases (solid, liquid, and gas) are in
equilibrium. Since the triple point is a point, there is only one temperature and one
pressure where the three phases will exist. This fact often helps in identifying compounds
or in problem solving.
The critical point is the highest temperature and pressure at which a
pure material can exist in vapor/liquid equilibrium. At temperatures higher than the
critical temperature, the substance can not exist as a liquid, no matter what the
pressure.
At temperatures and pressures higher than the critical point, the substance is
considered a fluid--something neither gas or liquid. At pressures lower than the critical
pressure (but at higher temps), the substance is considered a gas.
Calculations in 10.213 do not usually involve temperatures past the critical
temperature.
 The three phase equilibrium curves meet at the triple
point. At the triple point, all three phases (solid, liquid, and gas) are in
equilibrium. Since the triple point is a point, there is only one temperature and one
pressure where the three phases will exist. This fact often helps in identifying compounds
or in problem solving.
The three phase equilibrium curves meet at the triple
point. At the triple point, all three phases (solid, liquid, and gas) are in
equilibrium. Since the triple point is a point, there is only one temperature and one
pressure where the three phases will exist. This fact often helps in identifying compounds
or in problem solving.