A Primer on Chirality, Pseudo-Chirality, and Polymer Tacticity
Chirality
Some objects exhibit the property of "handedness" - the object and its mirror image are similar, but not identical. Our left and right hands are, to a first approximation, identical in almost every respect, but the right hand will not fit into a left-hand glove, and vice versa. The term which describes this "handedness" is chirality. A molecule that is not superposable with its mirror image is called chiral; a molecule that can be superposed in all three spatial dimensions with its mirror image is called achiral. Clearly, chiral molecules have two forms which are mirror-symmetric; these two molecules are called enantiomers, and are chemically similar, but structurally distinct, molecules.
For a molecule to be chiral, it must possess an asymmetric center, an atom (usualy a carbon) with four distinctly different groups bonded to it. This is a necessary, but not sufficient, condition for a molecule to be chiral, since molecules with more than two asymmetric centers can be achiral. Consider two molecules of 2-bromobutane:
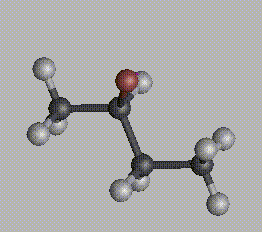
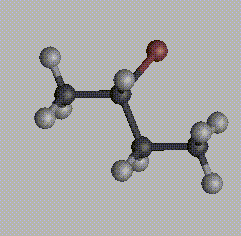
One is a mirror image of the other. No matter how you reorient these molecules in space, it is impossible to get an exact match. Try this for yourself, using your mouse to reorient the molecule below to match first one, and then the other, of these two images.
The Empirical Distinction between Enantiomers
Experimentally, two vials containing the pure enantiomers of a compound may be distinguished by the interaction of light with each enantiomer. In short, an enantiomer in its pure form is optically active, meaning that it has the effect of rotating the direction of polarization of a beam of plane polarized light which passes through it. This rotation is easily detected by placing a polarizing filter (the "polarizer") in front of the sample at fixed orientation, to polarize the incoming beam of light, and a second polarizing filter (the "analyzer") behind the sample, to detect the optical rotation. Only light waves oscillating in one direction will pass each filter. By rotating the second filter relative to the first, a maximum (when the analyzer is aligned parallel to the rotated polarized beam exiting the sample) or minimum (when the analyzer is aligned perpindicular) of light intensity can be detected. A compound which causes the plane of polarization to rotate in a clockwise (positive) direction is designated dextrorotatory, (+), while one which causes the polarization to rotate in a counterclockwise (negative) direction is designated levorotatory, (-). However, these empirical designations tell nothing about the actual structure of the molecules.
The Absolute Distinction between Enantiomers
To distinguish two enantiomers in an absolute sense, the R-S convention is used (R for "rectus" and S for "sinister"). To identify the absolute configuration of an enantiomer, follow these steps:
- Identify the asymmetric atom, and assign a "priority" to each of the groups bonded to it (a>b>c>d)
- Orient the molecule so that one looks down the bond from the asymmetric carbon to the group of lowest priority (d). The remaining substituents (a,b, and c) will be oriented in a circle around the asymmetric atom.
- Trace a path from a to b to c. If the path is clockwise about the asymmetric atom, the asymmetric atom is designated R; if it is counterclockwise, the atom is designated S.
The rules for establishing priority of different substituents are as follows:
- First, assign priority based on the atoms bonded directly to the asymmetric center such that higher atomic number has higher priority than lower atomic number.
- Second, for cases where two directly-bonded atoms are isotopes, assign priority to the one with higher atomic mass.
- Third, for cases where two substituents are the same atom, proceed outward to neighboring atoms in the substituent groups and assign priority based on the first point of difference. Where double or triple bonds are encountered, these are treated by assuming that each such bonded atom is duplicated or triplicated.
Based on these rules, what is the absolute configuration of each of the the three molecules shown above? (answer)
Pseudo-Chirality
In polymers, one encounters the situation where some asymmetric centers are pseudo-chiral. Rigourously speaking, the atoms are truly chiral, with four distinctly different substituents bonded to it. However, two of these substituents are the long arms of the polymer chain, each one often hundreds or thousands of atoms long, with the difference between the arms being only tens of atoms. These two substituents are of different molecular weight, but the difference is small compared to the total molecular weight of either arm. In this case, the atom is chiral in an absolute sense, but it does not result in a detectable rotation of plane polarized light. The chain molecule and its mirror image are "almost" superposable.
Polymer Tacticity
Many polymers are generated from monomers which, when polymerized, contribute an asymmetric atom to every repeat unit of the chain. Styrene itself is not chiral, but each repeat unit of the chain acquires a pseudo-chiral atom when it is polymerized to form polystyrene.
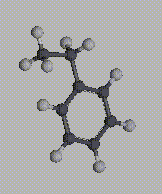
In this case, what becomes important is the sequence of absolute configurations along the backbone of the chain. The shortest distinguishing piece of the sequence is the diad, or two consecutive absolute configurations. If two consecutive configurations have the same absolute configuration (e.g. R-R or S-S), the diad is designated a meso diad; if they are different (e.g. R-S or S-R), the diad is designated a racemic diad. Since the absolute configurations are only "pseudo-chrial", R-R and S-S diads are "almost superposable" when the chain is rotated by 180 degrees, as are R-S and S-R diads. Polymer tacticity, then, is classified according the diad sequence. Two extreme cases are the following:
- Isotactic polymer consists entirely of meso diads. This means the absolute configuration sequence is either ...-RRRRRRR-... or ...-SSSSSSS-..., but the two are indistinguishable for all practical purposes. In fact, if one puts aside for the moment the distinct identity of each asymmetric atom, the two chains with different meso sequences may be exactly superposable!
- Syndiotactic polymer consists entirely of racemic diads. The absolute configuration sequence is ...-RSRSRSRS-.... Syndiotactic polymer typically have very different properties from their isotactic analogues.
- Of course, there remains a huge number of other possible sequences of R and S. The most usual case is one where the sequence is random. These polymers, and any polymer in which the sequence of absolute configurations is not highly ordered, is called an Atactic polymer.
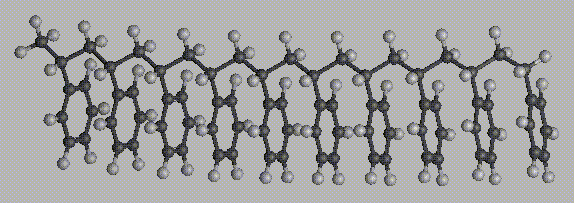
Three 10-repeat unit segments of polystyrene are illustrated here. The first is isotactic polystyrene, the second is syndiotactic polystyrene, and the third is neither (atactic polystyrene). Examples of meso diads are highlighted in blue, and examples of racemic diads are highlighted in red.
Back to reading list | Back to syllabus | Back to Course Notes
Last modified 3/10/99 - GCR





