Project Amazonia: Threats - Pollution
Acidification and pH
Acidification is a naturally occurring process in nature. In tropical areas with high rainfall, natural acidification of soils and surface waters is common. However, tropical areas are especially sensitive to further acidification by increased atmospheric deposition of sulfate and nitrate ions (Rodhe et al, 1988). The following describes the three conditions for an aquatic ecosystem to be acidified by atmospheric deposition:
· Atmospheric deposition of sulfate or nitrate or of some anion must increase.
· Adjacent soils to the aquatic ecosystem must not retain the anion that is increased in deposition.
· Aquatic ecosystem must have a low alkalinity for acidification to result in biological damage (Rodhe et al, 1988).
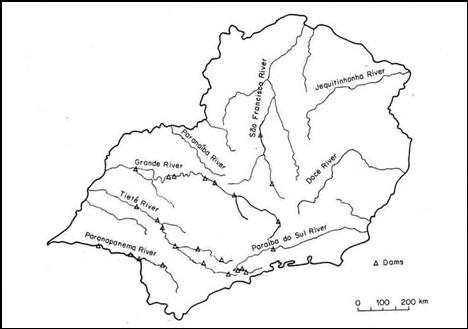
The major
rivers and tributaries (see map below) of the southeastern region of Brazil have
varying levels of pH. The tables give the measurements of pH, SO4-2,
and NH4+ for these rivers and their tributaries
(Moreira-Nordemann, 1988).
Figure 2: Rivers of Southeastern Brazil
Table 12: São Francisco River and Tributaries (T); minimum and maximum values based on one sample per year (1982-1983) at several points on each river
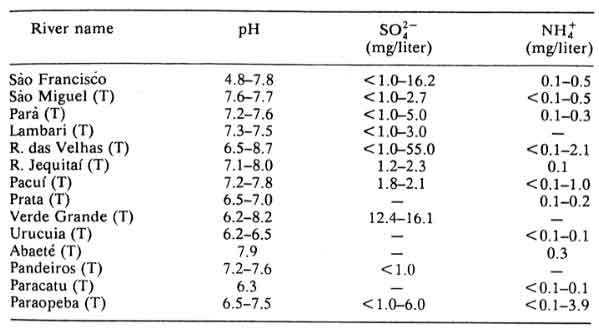
Table 13: Paraíba do Sul basin and tributaries (T), in Rio de Janeiro state in 1984
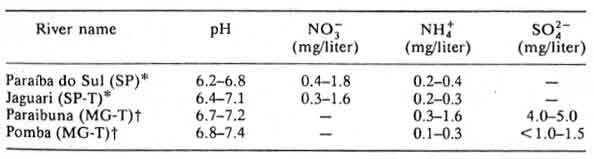
Table 14: Tieté River and tributaries (T); minimum and maximum
values obtained during 1981, 1983 and 1984 in monthly measurements
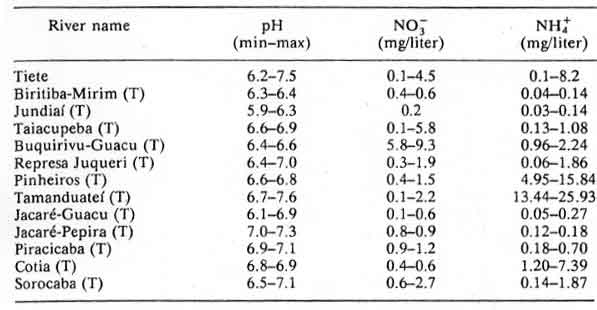
Table 15: Panapanema basin and tributaries (T), Sao Paulo state. Minimum and maximum mean values obtained for 1981, 1982 and 1983 in monthly measurements.
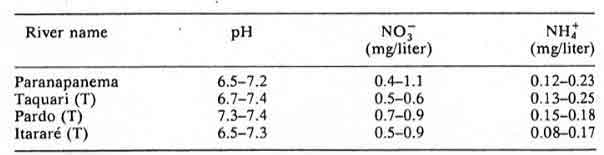
Table 16: Grande basin and tributaries, Sao Paulo state; minimum and maximum mean values obtained in 1981, 1982 and 1983 in monthly measurements.
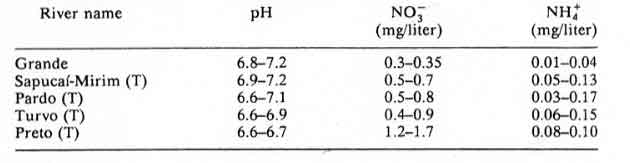
According to the authors of Chapter 8: Acidification in Southeastern Brazil,1 “The differences in nitrogen and sulfur concentrations observed in river waters of the southeastern region of Brazil cannot be explained by geological, pedological, or climatic factors. Higher NO3-, NH4+ and SO42- contents were determined in rivers crossing urban and industrial areas, the same areas that also present a polluted atmosphere.”
These increases may result from acid deposition. “Acid deposition” is caused by pollution from motor vehicles, industrial process, and the burning of fossil fuels in power-stations in the form of sulphur dioxide, nitrogen oxide, and hydrocarbons. These react with water and sunlight to form dilute sulphuric acid, nitric acid, ammonium salts, and other mineral acids2.
There are two types of “acid deposition” from the atmosphere: wet and dry (Fig. 3).
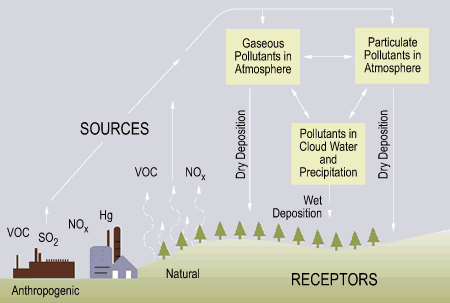
Figure 4: Acid deposition (EPA, 2002)
Wet deposition refers to acid rain, fog, and snow. According to the Environmental Protection Agency, “the strength of the effects [of acidic water] depends on a variety of factors, including how acidic the water is, the chemistry and buffering capacity of the soils involved, and the types of fish, trees, and other living things that rely on the water.”
Dry deposition refers to acidic gases and particles. Acidity in the atmosphere falls down as dry particles. These particles are deposited onto buildings and other structures, or are washed from trees and other surfaces by rain. This run off water adds acids to the acid rain, thereby increasing the acidity of the rain3.
Many organisms cannot tolerate high levels of acidity, and even those who can, face the problem that their food sources (such as insects) cannot survive in highly acidic environments. As acidity in a water system increases, the number and diversity of organisms decreases. Also, when acid rain flows through soils in a watershed, aluminum is released into the watershed, which is toxic to fish. At levels of pH5, most fish eggs cannot hatch4. Table 6 clearly shows that the effects of acidification on aquatic biota can be harmful.
Table 17: Effects of acidification on aquatic biota5
|
Physical and chemical changes |
|
||||||||
|
Primary production and Invertebrates |
|
||||||||
|
Responses of Fish Populations to Acidification |
|
Because the water system of the Amazon is such a large and complex one, it is difficult to understand the true nature of acidity and acid deposition’s effects on the water although there are clearly relationships between changes in the acidity of water and pollution.
HUMAN ALTERATION OF THE NITROGEN CYCLE
Most of the human activities responsible for the increase in global nitrogen are local in scale, from the production and use of nitrogen fertilizers to the burning of fossil fuels in automobiles, power generation plants, and industries.
Nitrogen Fertilizers
Industrial fixation of nitrogen for use as fertilizer currently totals approximately 80Tg per year and represents the largest human contribution of new nitrogen to the global cycle. This figure does not include manure and other organic nitrogen fertilizers. (8) The use of fertilizer application in developed countries has stabilized. However, it has risen drastically for developing countries. Human population growth and increasing urbanization ensures that the rate of industrial fertilizer production will continue to accelerate for decades in order to meet the escalating demand for food. This scenario is the present case in the Amazon Basin Rainforest Ecosystem (ABRE). Due to migration and other factors, the indigenous population in the ABRE is currently rising. In addition, improved transportation is attracting more migrants into the area. Their destruction of rainforest land and their use of fertilizers further impacts the nitrogen cycle.
Fossil Fuel Burning
The burning of fossil fuels (i.e. coal and oil) releases previously fixed nitrogen back to the atmosphere in the form of nitrogen-based trace gases such as nitric oxide. High- temperature combustion also fixes a small amount of atmospheric nitrogen directly. Together, the operation of automobiles, factories, power plants, and other combustion processes emit more than 20 Tg per year of fixed nitrogen to the atmosphere.
Mobilization of Stored Nitrogen
Besides enhancing fixation and releasing nitrogen from geological reservoirs, human activities, such as burning of forests, wood fuels, and grasslands also liberate nitrogen from long- term biological storage pools such as soil organic matter and tree trunks, contributing further to the release of biologically available nitrogen. One of the major consequences of human- driven alterations in the nitrogen cycle has been regional and global changes in the chemistry of the atmosphere- specifically increased emissions of nitrogen- based trace gases including nitrous oxide, nitric oxide, and ammonia. If left unattended, these gases can have detrimental long term effects. Nitrous oxide contributes to the greenhouse affect while nitric oxide is an important precursor of acid rain and photochemical smog.
Nitrous oxide is a very effective heat- trapping gas in the atmosphere. This is in part because it absorbs outgoing radiant heat from the Earth in infrared wavelengths that are not captured by other major greenhouse gases. Although it is fairly unreactive in the lower atmosphere, when it rises into the stratosphere, it can trigger chain reactions that deplete and thin the stratoshperic ozone layer that shields the Earth from damaging ultraviolet radiation.
Both nitric oxide and ammonia are highly reactive in the lower atmosphere. Nitric oxide plays several crucial roles in atmospheric chemistry, including catalyzing the formation of photochemical smog. In the presence of sunlight, nitric oxide and oxygen react with hydrocarbons emitted by automobile exhausts to form ozone- the most dangerous component of smog. Ground- level ozone has serious detrimental effects on human health as well as the health and productivity of crops and forests. Nitric oxides, along with other nonmetal oxides, can be transformed in the atmosphere into nitric acid and sulfuric acid (for example), which are major components of acid rain.
Among these many sources of nitric oxide emissions, combustion is the dominant one. A chief danger of the increasing levels of nitrogen is the threat that it poses to the carbon cycle. Experiments in Europe and America indicate that a large portion of the extra nitrogen retained by forest, wetland, and tundra ecosystems stimulates carbon uptake and storage (can you add specific details of the project: name of project, who ran the experiment, etc and citations?). On the other hand, this nitrogen can also stimulate microbial decomposition and thus releases of carbon from soil organic matter. On balance, however, the carbon uptake through new plant growth appears to exceed the carbon losses, especially in forests. The most recent analysis of the global carbon cycle by the Intergovernmental Panel on Climate Change concluded that nitrogen deposition could represent a major component of the missing carbon sink.
Nitrogen Saturation
There is a limit to how much plant growth can be increased by nitrogen fertilization. Eventually, when the natural nitrogen supplies are replenished, plant growth becomes limited by scarcity of other nutrients, such as phosphorus, calcium, and water. When the vegetation can no longer respond to further additions of nitrogen, the ecosystem reaches a state described as nitrogen saturation. When an ecosystem is fully nitrogen- saturated and its soils, plants, and microbes cannot use or retain any more, all new nitrogen deposits will be dispersed to streams, groundwater, and the atmosphere6.
Nitrogen saturation has a number of damaging consequences for the health and functioning of ecosystems. These effects were first observed in Europe when scientists noticed a large increase in nitrate concentration in some lakes an streams. As ammonium builds up in the soil, it is increasingly converted to nitrate by bacterial action. This process releases hydrogen ions and helps acidify the soil. The buildup of nitrate enhances emissions of nitrous oxides from the soil and also encourages leaching of highly water- soluble nitrate into streams or groundwater. As these negatively charged nitrates seep away, they carry with them positively charged alkaline minerals such as calcium, magnesium, and potassium. This in turn alters the soil composition and depletes it of other nutrients necessary for healthy plant growth. As calcium is depleted and the soil acidified, aluminum ions are mobilized, eventually reaching toxic concentrations that can damage tree roots or kill fish if the aluminum washes into streams. The trees are starved of calcium, magnesium, and potassium, a nutrient imbalance arises in their roots and leaves. This can potentially reduce the photosynthetic rate and efficiency of the plant, stunt its growth, and even increase tree death.6
Nitrogen saturation plays a deeper role and also influences biodiversity, species mix, and aquatic ecosystems.33
Soil Contamination
Soil contamination is the mixing of hazardous substances with the soil. These contaminants get physically or chemically attached to the soils or are trapped within its particles. This contamination is one of the natural results of extensive land use which occurs in the Amazon Basin Rainforest Area. The main types of soil contamination in the Amazon Basin Rainforest are from mercury, cyanide and contamination from pesticides.
Mercury
Mercury contamination is found in many areas of the Amazon basin especially along the Tapajos River where gold mining is carried out by an estimated million miners. For several years it was believed that the mercury in the rivers of the Amazon came solely from the mining operations conducted by the miners. Miners use mercury to clean the gold that they extract from the river, and due to lack of interest in environmental matters, and a lack of education, much of this mercury is released into the environment, some into the soil, some into the waters, but most is sent to the atmosphere, returning to the earth in rain.
To clean the gold, the miners mix the silted-gold with mercury which separates the gold from the silt. The mercury is then burned off the gold, at which point it evaporates. Mercury vapor is subsequently sent to the atmosphere. There have been attempts to educate these miners on the use of fume cupboards to prevent the excess mercury from escaping to the atmosphere, and on alternative gold-cleaning methods, but the majority still utilizes mercury without fume cupboards.
Though most of this mercury contamination is found in the river water, some of it eventually travels to the land in the form of silted soil deposited on river plains during flooding. Mercury contamination also results from the exposure of naturally occurring deposits in locations where a lot of vegetation is lost during deforestation. Continuous loss of vegetation accelerates the erosion, which in turn wears away the earth and reaches the underlying mercury layers.
Recent studies6 show that mercury from mining operations contributes a small percentage of the total mercury found in the Amazon. Research demonstrates that mercury levels at several hundred kilometers down stream mining operations are in the same range as levels of mercury a few kilometers downstream7. Mercury then has more than one source in the Amazon, and has increased in the last forty or so years. The scientists have linked the increase in Mercury with the initiation of slash-and-burn agriculture which is prevalent in the Amazon today.
By removing trees, loggers and 'slash and burn' farmers remove that which holds the soil together. In the soil are natural accumulating Mercury deposits bounded to soil particles. These deposits are unavailable for organisms as the mercury is in its inorganic form. When deforestation occurs, the exposed soil is easily washed away by rains into the water ways. This is the point where mercury becomes poisonous, by separating from the soil particles, the mercury is converted to its organic form called methylmercury by the equation:
Hg+ + CH3- => CH3Hg
Mercury concentrations in the rivers of the Amazon have been estimated at 0.0002ppm8. Methylmercury is extremely poisonous, and in that form, it can be absorbed by fish and other aquatic fauna9, and travel up the food-chain. Accumulation will occur, where the concentrations of mercury in organisms higher in the food chain increases as they consume the lower organisms. Mercury concentrations in humans can get to be several magnitudes greater than their previous concentrations by having mercurial fish in their diet. The locals living along the Amazon rivers are therefore at risk of mercury poisoning. The miners are experiencing Mercury Poisoning or Miramata Disease as well.
The main source of cyanide contamination is gold mining operations as well. A notable site of this pollution is the Omai gold mines in Guyana to the north of Brazil.
Pesticides
Pesticide contamination is most prevalent in areas where agriculture is used. Although agriculture is not responsible for as much deforestation as cattle ranching, the effects are still extensive. The immigrants from the cities are ill equipped with agricultural and environmental conservation skills and so use chemicals of various kinds recklessly to reduce weed and insect infiltration to their crops.
The effects of the pollution on soil are observed in many ways. Soil contamination makes it largely impossible for healthy vegetation to grow on the affected land. When the plants absorb these contaminants through their roots, they either develop weak stems, deformed leaves, or reproductive failures. Some of the contaminants also slow the growth and development of the vegetation making recovery in areas where the forest has been cleared ineffective. Animals in the rain forest are also impacted by these adverse effects. Burrowing animals come into direct contact with the mercury in the soil. The interaction with the chemicals interferes with respiratory processes and causes brain damage. Both effects lead to immature deaths of these animals. Some of the soil contaminants, especially mercury and cyanide, are carried in the air due to wind erosion and are then inhaled by the fauna in varying quantities. Additionally, animals absorb contaminants when they feed on contaminated vegetation.
Nitrogen Oxides
Background:
Nitrogen oxides are emitted by the burning of fuels, by industry, and through natural (nitrogen cycle) processes. They cause ozone depletion and acid rain and are short-lived in the atmosphere Nitrogen oxides are reactive, greenhouse gases, including NO and NO210. When released into the atmosphere through natural (nitrogen cycle) and artificial sources (combustion of fuel, industry), these compounds enter a complex reactionary period of a few days. The main products of this reaction period are tropospheric ozone (O3) and nitric acid (HNO3).11
NO2 + hv (wavelength less than 410 nm) à NO + O
O + O2 + catalyst à O3 + catalyst
NO + O3 à NO2 + O2
And
R + O2 + catalyst à RO2 + catalyst
RO2 + NO à NO2 + RO
These reactions show the creation of ozone gas and its concurrent consumption. However, in the presence of organic molecules of solely carbon and hydrogen (R), NO favors the second group of reactions and the ozone is not re-consumed. This process creates high levels of tropospheric ozone.
A self cleaning process is constantly breaking NOx molecules into nitric acid (HNO3) through reaction with hydroxyl radicals.
HO + NO2 + catalyst à HNO3 + catalyst
Nitric acid is unreactive in the gas phase, but quite soluble in water(Graedel 152). Thus the acid concentrates in water droplets. The resulting acidic rain is damaging to soil processes and at very low pH can directly damage flora and fauna12.
Relevance to Rain Forest
Due to the short life of these compounds in the troposphere, areas of concern for the preservation of the rainforest are industrial and urban sites near or within the rainforest and the burning of the rainforest. All other sources of NOx will be filtered out of the atmosphere by natural processes long before reaching the Amazon River Basin Rainforest ecosystem.
Sulfur Compounds
Background:
Atmospheric
sulfur is critical to atmospheric acid-base chemistry. However, in more recent
times, human industry has thrown the sulfur cycle out of balance, leading
directly to acidic rain and increasing aerosol levels13. The main
atmospheric sulfur compounds are sulfates: sulfuric acid, ammonium hydrogen
sulfate, and ammonium sulfate.
The following reactions occur in the troposphere:
HSO3- + H2O2 --> HSO4- + H2O
HSO3-+ O3 --> HSO4- + O2
While this reaction appears to be beneficial through the decomposition of tropospheric ozone, the product HSO4- quickly forms H2SO4, or sulfuric acid. This compound, along with nitric acid, dissolves into water droplets, concentrates in clouds, and results in acidic rain14.
Relevance to Rain Forest:
Sulfur, as
with Nitrogen, plays its damaging role not in the atmosphere itself, but once it
is converted into water soluble compounds which collect in water droplets within
clouds. These compounds are deposited by rainfall and cause damage to plant
life, animal life, and water sources.
Sulfur
Background:
Sulfur dioxide and hydrogen sulfide both are toxic gases that can form from elemental sulfur in the atmosphere. They are emitted by the use of Diesel gas and by coal burning power plants. Sulfur also causes ozone depletion and acid rain. However, sulfur also causes some global cooling and function as a nutrient to some plants. It helps them form proteins and aids photosynthesis. A lack of sulfur would impede growth in at least some plants.15
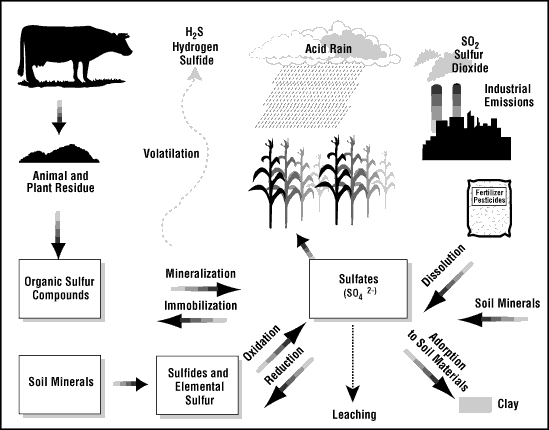 16
16
In conditions with abundant sulfur, plants incorporate sulfur into organosulfur and involve sulfur in energy transfers. Below is an example of this in Chlorella.
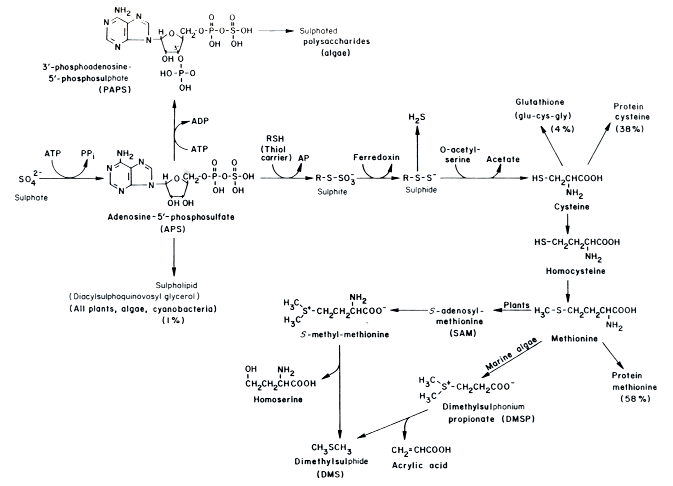 17
17
Obviously, sulfur can be an important part of algae and plants’ metabolic pathways.
According to the Iris Hypothesis, availability of Sulfur is also important in counteracting the greenhouse effect. Excess sulfur in the atmosphere called aerosols forms clouds that are effective in reflecting sunlight, causing global cooling18,19. (More information about the Iris hypothesis is given in research regarding climate.)
Recent sulfur emissions:
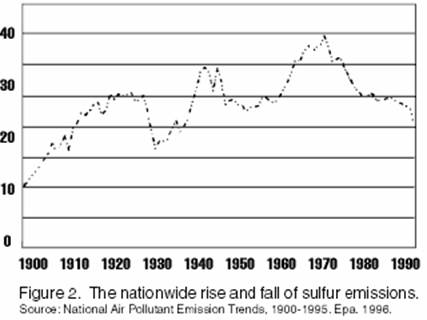 20
20
Ozone:
The ozone layer is a layer in the upper atmosphere protecting us from the sun's harmful UV rays which are harmful to many plants and animals. Ozone is made thru the combination of molecular oxygen and atomic oxygen and ozone depletion is caused by many of the compounds that also form acid rain.
NO + O3 --> NO2 + O2 k = 1.8e-15 at 300 K
SO + O3 --> SO2 + O2
The main source of SO in the atmosphere is oxidation of COS, which is produced through burning of fossil fuels.
OH + O3 --> O2 + HO2
HO2 + O --> OH + O2
Net reaction: O3 + O --> 2O2
O3 + Cl --> O2 + ClO
ClO + O --> O2 + Cl
Net reaction21: O3 + O --> 2O2
Similar reactions will occur with other halogens. The harmfulness of elements in the atmosphere depends on their lifetime in the atmosphere. As seen above, aerosols are a source of ozone depletion even though they block the sun themselves22. The effects of bromine in particular are much greater than those of chlorine because of bromine’s long lifetime.23 Below are the relative lifetimes of some ozone depleting compounds.24
| Compound | Lifetime |
| CO2 | 100-250 |
| CH4 | 8 |
| CFC-11 | 65 |
| CFC-12 | 120 |
| N2O | 150 |
| C2Cl2F3 | 90 |
| CH3CCl3 | 6 |
At this point however, the ozone problem is under more control due to regulations like the Montreal Protocol,25,26 so the depletion of the ozone layer is not a major threat to the health of plants and animals in the Amazon rainforest.
Climate
Many things can cause a change in climate, but one of the major controllable factors disrupting climate patterns is deforestation. When an area of trees is cleared, the humidity in that region decreases because trees aren’t there to circulate moisture through the soil. This results not only in a drier region near the vicinity of the deforestation but also in wetter regions thousands of miles away from the area that was deforested.27 This is because normally rain is formed through water vapor condensation, which releases heat. Heat causes convection, the rising of air due to its high temperature. The effect is global because warm air can travel far distances and land thousands of miles away. With decreased humidity and convection in the Amazon, places as far as the US may experience increased precipitation.28
There are two hypotheses that predict the results of having less humidity and more heat in the atmosphere. The first suggests that more dispersed, thin clouds will form and will be able to block the sun better than thicker clouds because they are able to cover more area. The result of this is global cooling.29 The other suggests that clouds in a warmer region will be smaller due to lack of moisture and will cover less area, blocking less sun. The second phenomenon is more supported and is called the Iris effect (see pollution).
However, the Iris effect is still optimistic and hypothesizes that these clouds will cause global cooling rather than warming. It suggests that the thinner and more spread out clouds in the atmosphere will also be more efficient at releasing heat.30 It argues that the release of heat by these clouds is much more than the extra radiation from the sun let in by these clouds so the net effect is global cooling.31 This would be a great negative feedback system for climate control. However, results from the Tropical Rainfall Measuring Mission (TRMM) satellite launched by NASA suggest the opposite. Clouds and the Earth’s Radiant Energy System has modeled the exchange of energy and has found that the amount of heat let in by these clouds is much more than the amount of heat that escapes due to these clouds. Thus, the net effect is actually more global warming.32
Next: Agriculture and Cattle Ranching ->
![]()
1: L. M. Moreira-Nordemann, M. C. Forti, V. L. Di Lascio, C. M. do Espirito Santo and O. M. Danelon. "Chapter 8: Acidification in Southeastern Brazil." Acidification in Tropical Countries. Ed. Rafael Herrera, and Henning Rodhe. N.p.: John Wiley & Sons, 1988.
2: Mayhew, 1997
3: EPA, 2002
4: EPA, 2002
5: Mills, 1984
6: Lebel, Jean, “Mercury Poisoning in the Amazon: The tip of the Iceberg”, IDRC. http://www.idrc.ca/Media/MercuryPoisoning_e.html, Nov 26, 2002
7: Lebel, Jean, “Mercury Poisoning in the Amazon: The tip of the Iceberg”, IDRC http://www.idrc.ca/Media/MercuryPoisoning_e.html, Nov 26, 2002
8: Maruyama, Sadao, “Tracing Mercury in the Amazon”, The Rigaku Journal, volume 15, no. 1, 1998
9: Maruyama, Sadao, “Tracing Mercury in the Amazon”, The Rigaku Journal, volume 15, no. 1, 1998
11: Graedel, T. E., Atmospheric Change: An Earth System Perspective. W. H. Freeman and Company, 1993.
12: Mayer, Robert; Liess, Siegfried; et al. Atmospheric Pollution in a Tropical Rain Forest: Effects of Deposition upon Biosphere and Hydrosphere. Environmental Engineering Abstracts, 16 August 1999.
13: http://www.pmel.noaa.gov/pubs/outstand/bate1229/intro.shtml
14: Graedel, T. E., Atmospheric Change: An Earth System Perspective. W. H. Freeman and Company, 1993.
21: Brimblecombe, Peter. Air Composition and Chemistry. Cambridge Environemntal Chemistry series 6. Cambridge University Press, 1986. p44, 201, 120, 193.
22: Brimblecombe, 201.
23: Makhijani, Arjun and Kevin R. Gurney. Mending the Ozone Hole: Science, Technology and policy. Institute for Energy and Environmental Research. The MIT Press, 1995. p180-185
24: Brimblecombe, 187
29: Conversation with Jeremy Boyce.
32: http://earthobservatory.nasa.gov/Study/Iris/iris2.html
33: http://esa.sdsc.edu/tilman.htm
