Air CompoundsSulfur
Overview:Carbon Dioxide
- emitted by the used of Diesel gas
- emitted also by coal burning power plants
- causes ozone depletion and acid rain
- causes some global cooling
- could be a soil nutrient
Background:Recent Sulfur Emissions:Sulfur dioxide and hydrogen sulfide both are toxic gases that can from from elemental sulfur in the atmosphere. Sulfur compounds in the atmosphere can form acid rain, which is harmful to plants. Ironically, sulfur is also a nutrient to some plants. It helps them form proteins and aids photosynthesis. A lack of sulfur would impeded growth in some plants.(Nutrient Manager, 3)
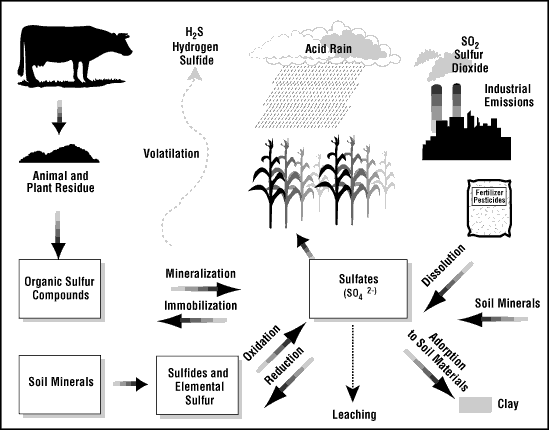
(Nutrient Manager, 1)In conditions with abundant sulfur, plants incorporate sulfur into organosulfur and involve sulfur in energy transfers.
According to the Iris Hypothesis, availability of Sulfur is also important in counteracting the greenhouse effect. Excess sulfur in the atmosphere called aerosols forms clouds that are effective in reflecting sunlight, causing global cooling. The theory is that sulfur forms compounds with the water molecules in clouds, bring the molecules closer and thus making the clouds denser. This makes them able to reflect light from the sun better. (GSReport, 1 + enn news, 1)
However, other sources say that these brighter, denser clouds scatter and absorb the sun's radiation better, leading to more heat in the atmosphere and less heat reaching the earth. In addition, rainfall decreases since these clouds are not as efficient in releasing water. This causes concern over fresh water resources. (Ramanathan, 1) Thus is it is uncertain what the effects of sulfur on climate are.
SourcesGiven the toxicity of sulfur, this is not a good trend. However, in the rainforest, the increase of sulfur has not had big effects.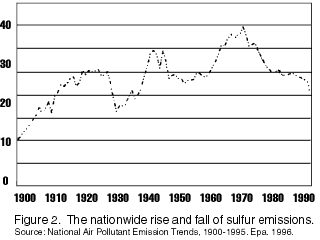
(Nutrient Manager, 2)
We have determined that acid rain is not actually a large influence in the rain forest for the following reasons: 1) acid rain is generally produced through excess nitrgen and sulfur emission from industrial sites. No industrial sites are in or so near the amazon rainforest as to have an effect. 2) Collaboration with other groups has informed us that the soil in the rainforest is naturally acidic which implies that some acid in the rain is good for the rainforest. Thus, we have concluded that acid rain is not a influential factor in the well being of the rainforest.1) Nutrient Manager: Volume 5, Number 1, Summer 1998
http://www.agnr.umd.edu/users/agron/nutrient/Factshee/sulfur/Sulfur.html2) http://www.gsreport.com/articles/art000175.html
3) http://www.enn.com/news/enn-stories/1999/07/071999/sulfur_4414.asp
4) Ramanathan, V., P.J. Crutzen, J.T. Kiehl, D. Rosenfeld. Aerosols, Climate, and the Hydrological Cycle. Science's Compass: Vol 294, Dec 7 2001. p2119-2124
OzoneDepending on the climate and the season, the rainforest may act as a sink or a source for carbon dioxide. El Nino and La Nina, in particular change the emission/absorption of carbon dioxide significantly. On average, in the past two decades, the rainforest has acted as a sink for carbon dioxide.
Current records of the carbon cycle suggest that there is a missing carbon sink taking carbon away from the atmosphere.The recent trend in world Carbon Dioxide levels is shown:
It is steadily increasing, which is cause for alarm. However, the missing carbon sink is definitely a mystery that has to be solved before a truly effective plan is reached on how to solve the problem of increasing carbon dioxide levels.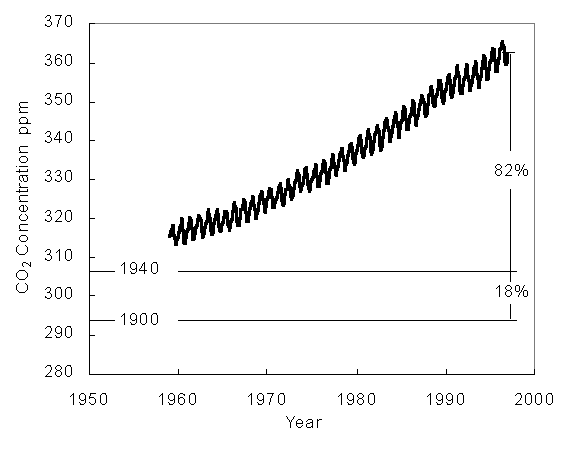
(http://www-personal.umich.edu/~mnwalsh/amazon.html)Source: Walsh, Molly, Melissa Maxwell, Elizabeth Zorza. Is the Amazon Basin a Source or a Sink for Carbon Dioxide? http://www-personal.umich.edu/~mnwalsh/amazon.html
The ozone layer is a layer in the upper atmosphere protecting us from the sun's harmful UV rays which are harmful to many plants and animals. Ozone is made thru the combination of molecular oxygen and atomic oxygen and ozone depletion is caused by many of the compounds that also form acid rain.NO + O3 --> NO2 + O2 k = 1.8e-15 at 300 K
SO + O3 --> SO2 + O2
The main source of SO in the atmosphere is oxidation of COS, which is produced through burning of fossil fuels.OH + O3 --> O2 + HO2
HO2 + O --> OH + O2
Net reaction: O3 + O --> 2O2O3 + Cl --> O2 + ClO
ClO + O --> O2 + Cl
Net reaction: O3 + O --> 2O2 (Brimblecombe, p44, 201, 120, 193)Similar reactions will occur with other halogens.
Halons are also compounds that deplete the ozone layer. They were originally used for firefighting. Currently, they are used more for fire protection in electronics where water would seriously harm the equipment. Halons contain mainly Bromine and sometimes Chlorine. Bromine is oxidized by ozone(and thus depletes it) the same way that Chlorine is oxidized by ozone. However, Bromine is much more effective because it can stay around in the atmosphere for a much longer amount of time. Thus, there is little Bromine available in the atmosphere, the damage they cause is comparable to that caused by Chlorine in the atmosphere. (Makhijani, p180-184)
The harmfulness of elements in the atmosphere depend on their lifetime in the atmosphere. As seen above, aerosols are a source of ozone depletion even though they block the sun themselves. (Brimblecombe, 201) The effects of bromine in particular are much greater than that of chlorine because of bromine's long lifetime. (Makhijani, 180-5) Below are the relative lifetimes of some ozone depleting compounds.(Brimblecombe, 187)
CO2 .............................................................................................. 100-250
CH4 ............................................................................................... 8
CFC-11 .............................................................................................. 65
CFC12 .............................................................................................. 120
N2O .............................................................................................. 150
C2Cl2F3 .............................................................................................. 90
CH3CCl3 .............................................................................................. 6In this case, the lifetime of CO2 is 100-250 relative to other compounds.
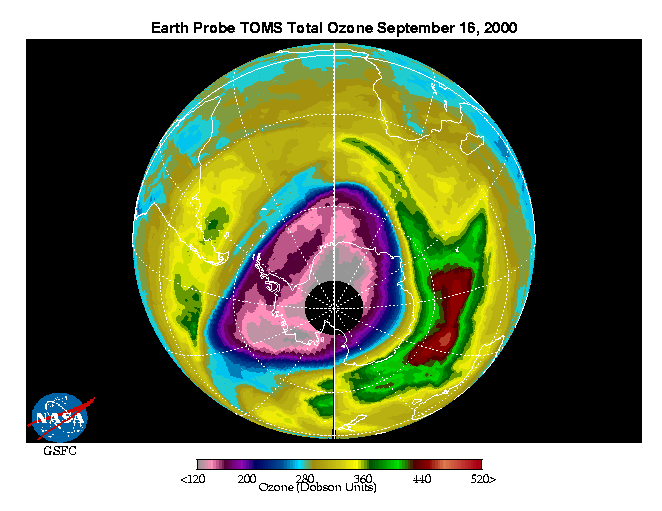
(http://jwocky.gsfc.nasa.gov/multi/recent_ozone91200.gif)
At this point however, the ozone problem is very much in control due to regulations like the Montreal Protocol(source:TC and AMP). In particular, the Copenhagen Amendments to the Montreal Protocol
limited CFC(chloroflurocarbon, a type of halocarbon, hazardous to the ozone as shown below(Makhijani, p93-4)) emissions. The Copenhagen Amendment also limited allowed halon emissions. In particular, major nations were to stop using Halons in January 1994. (Makhijani, p180)Thus the depletion of the ozone layer currently isn't a major threat to the health of plants and animals in the Amazon rainforest.
Sources1) Tweflth Congress of the Federated States of Micronesia Second Regular Session(TC), 2001 C.R. NO. 12-15 http://www.fsmcongress.org/12congress/pdf/CR12.015.pdf2) Makhijani, Arjun and Kevin R. Gurney. Mending the Ozone Hole: Science, Technology and policy. Institute for Energy and Environmental Research. The MIT Press, 1995.
3) Brimblecombe, Peter. Air Composition and Chemistry. Cambridge Environemntal Chemistry series 6. Cambridge University Press, 1986.
4) Amendment to the Montreal Protocol. (AMP)
http://www.unep.org/ozone/Copenhagen-Amendment.shtml