Acidification
is a naturally occurring process in nature.
In high rainfall tropical areas, natural acidification of soils and
surface waters is common. However,
tropical areas are especially sensitive to further acidification by increased
atmospheric deposition of sulfate and nitrate ions (Rodhe
et al, 1988). The following describes
the three necessary conditions for the acidification of an aquatic ecosystem by
atmospheric deposition:
1) Atmospheric deposition of sulfate or
nitrate or of some anion must increase.
2) Adjacent soils to the aquatic
ecosystem must not retain the anion that is increased in deposition.
3) Aquatic ecosystem must have a low
alkalinity for acidification to result in biological damage (Rodhe et al, 1988).
The major
rivers and tributaries of the southeastern region of
Figure 2: Rivers of Southeastern Brazil

Table 1:
|
River Name |
pH |
SO42- (mg/L) |
NH4+ (mg/L) |
|
Sΰo Francisćo |
4.8 7.8 |
< 1.0 16.2 |
0.1 - 0.5 |
|
|
7.6 7.7 |
< 1.0 2.7 |
< 0.1 0.5 |
|
Parΰ (T) |
7.2 7.6 |
< 1.0 5.0 |
0.1 0.3 |
|
Lambari (T) |
7.3 7.5 |
< 1.0 3.0 |
-- |
|
R. das Velhas
(T) |
6.5 8.7 |
< 1.0 55.0 |
< 0.1 2.1 |
|
R. Jequitai (T) |
7.1 8.0 |
1.2 2.3 |
0.1 |
|
Pacuν (T) |
7.2 7.8 |
1.8 2.1 |
< 0.1 1.0 |
|
Prata (T) |
6.5 7.0 |
-- |
0.1 0.2 |
|
Verde Grande (T) |
6.2 8.2 |
12.4 16.1 |
-- |
|
Urucuia (T) |
6.2 6.5 |
-- |
< 0.1 0.1 |
|
Abaetι (T) |
7.9 |
-- |
0.3 |
|
Pandeiros (T) |
7.2 7.6 |
< 1.0 |
-- |
|
Paracatu (T) |
6.3 |
-- |
< 0.1 0.1 |
|
Paraopeba (T) |
6.5 7.5 |
< 1.0 6.0 |
< 0.1 3.9 |
Table 2: Paraνba do Sul basin and tributaries (T), in
|
River Name |
pH |
NO3- (mg/L) |
NH4+ (mg/L) |
SO42- (mg/L) |
|
Paraνba do Sul (SP)* |
6.2 6.8 |
0.4 1.8 |
0.2 0.4 |
-- |
|
Jaguari (SP-T)* |
6.4 7.1 |
0.3 1.6 |
0.2 0.3 |
-- |
|
Paraibuna (MG-T) |
6.7 7.2 |
-- |
0.3 1.6 |
4.0 5.0 |
|
Pomba (MG-T) |
6.8 7.4 |
-- |
0.1 0.3 |
< 1.0 1.5 |
Table 3:
|
River Name |
pH |
NO3- (mg/L) |
NH4+ (mg/L) |
|
|
6.2 7.5 |
0.1 4.5 |
0.1 8.2 |
|
Biritiba-Mirim (T) |
6.3 6.4 |
0.4 0.6 |
0.04 0.14 |
|
|
5.9 6.3 |
0.2 |
0.03 0.13 |
|
Taiacupeda (T) |
6.6 6.9 |
0.1 5.8 |
0.13 1.08 |
|
Buquirivu-Guacu (T) |
6.4 6.6 |
5.8 9.3 |
0.96 2.24 |
|
Represa Juqueiri (T) |
6.4 7.0 |
0.3 1.9 |
0.06 1.86 |
|
Pinheiros (T) |
6.6 6.8 |
0.4 1.5 |
4.95 15.84 |
|
Tamanduatei (T) |
6.7 7.6 |
0.1 2.2 |
13.44 25.93 |
|
Jacare-Gaucu (T) |
6.1 6.9 |
0.1 0.6 |
0.05 0.27 |
|
Jacare-Pepira |
7.0 7.3 |
0.8 0.9 |
0.12 0.18 |
|
|
6.9 7.1 |
0.9 1.2 |
0.18 0.70 |
|
Cotia (T) |
6.8 6.9 |
0.4 0.6 |
1.20 7.39 |
|
|
6.5 7.1 |
0.6 2.7 |
0.14 1.87 |
Table 4: Panapanema basin and
tributaries (T),
|
River Name |
pH |
NO3- (mg/L) |
NH4+ (mg/L) |
|
Paranapanema |
6.5 7.2 |
0.4 1.1 |
0.12 0.23 |
|
Taquari (T) |
6.7 7.4 |
0.5 0.6 |
0.13 0.25 |
|
Pardo (T) |
7.3 7.4 |
0.7 0.9 |
0.15 0.18 |
|
Itarare (T) |
6.5 7.3 |
0.5 0.9 |
0.08 0.17 |
Table 5: Grande basin and tributaries,
|
River Name |
pH |
NO3- (mg/L) |
NH4+ (mg/L) |
|
Grande |
6.8 7.2 |
0.3 0.35 |
0.01 0.04 |
|
Sapucai-Mirim |
6.9 7.2 |
0.5 0.7 |
0.05 0.13 |
|
Pardo |
6.6 7.1 |
0.5 0.8 |
0.03 0.17 |
|
Turvo |
6.6 6.9 |
0.4 0.9 |
0.06 0.15 |
|
Preto |
6.6 6.7 |
1.2 1.7 |
0.08 0.10 |
According
to the authors of Chapter 8: Acidification in Southeastern Brazil,
The differences in nitrogen and sulfur concentrations observed in river waters
of the southeastern region of
These increases
may be caused by acid deposition. Acid
deposition is caused by pollution from motor vehicles, industrial process, and
the burning of fossil fuels in power-stations, releasing sulfur dioxide,
nitrogen oxide, and hydrocarbons. These
pollutants react with water and sunlight to form dilute sulfuric acid, nitric
acid, ammonium salts, and other mineral acids (Mayhew, 1997).
There are
two types of acid deposition from the atmosphere: wet and dry (Fig. 2).
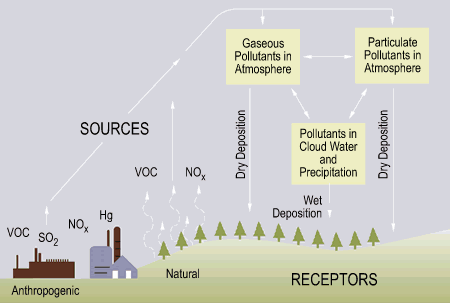
Figure 3: Acid deposition (EPA, 2002)
Wet
deposition refers to acid rain, fog and snow.
According to the Environmental Protection Agency, the strength of the
effects [of acidic water] depends on a variety of factors, including how acidic
the water is, the chemistry and buffering capacity of the soils involved, and
the types of fish, trees, and other living things that rely on the water.
Dry
deposition refers to acidic gases and particles. Ions in the atmosphere fall down as dry
particles. These particles are deposited
onto buildings and other structures, or are washed from trees and other surfaces
by rain. This runoff water exaggerates
the acidity of acid rain (EPA, 2002).
Many
organisms cannot tolerate high levels of acidity, and furthermore, many of the
species that are able to tolerate the increased acidity are faced with
diminished food supplies due to the increased acidic conditions. As acidity in a water system increases, the
number and diversity of organisms decreases.
Also, when acid rain flows through soils in a watershed, aluminum, which
is toxic to fish, is released into the water system. At a pH of 5, most
fish eggs cannot hatch (EPA, 2002). Table 10 details the harmful effects
of acidification on aquatic biota.
Table 6: Effects
of acidification on aquatic biota (Mills, 1984)
|
Physical and chemical changes |
· Water transparency has
increased, along with rates of hypolimnetic heating
and thermo cline deepening · Concentrations of Mn, Na, Zn, H+, S2O4-,
Al increased · Aluminum has been
implicated as a major cause of fish mortality during lake acidification · S2O4-
was reduced by bacteria to sulfide, followed by permanent sedimentation as |
|
Primary production and Invertebrates |
·
Primary production has increased in ·
Phytoplankton species composition has changed with Chlorophyceae and Peridineae
replacing Chrysophyceae ·
Appearance of hypolimnetic algal
·
Three members of the zooplankton community Mysis relicta, Epischura lacustris, Diaptomus sicilis disappeared
as pH declined to 5.4, while Daphnia catawba x schoedleri appeared |
|
Responses of Fish Populations to Acidification (pH lowered by 6.7 to 5.1) |
· The fathead minnow population
declined rapidly and almost disappeared when pH was 5.6. In addition,
complete reproductive failure, rapid collapse of population were observed · The pearl dace population rapidly
expanded to become the major minnow species when pH was 5.4. This was
probably due to its greater tolerance to low pH by pearl dace than fathead
minnow · White sucker (seen as relatively
acid-tolerant species) showed no stress as the pH of the lake was lowered.
Its individual fish growth remained consistently high. · The population of lake trout fish,
which are relatively acid sensitive, decreased when pH was lowered from 6.7
to 5.4. However, its population did not decrease at the rate which was
expected - it was much slower. |
Because the water system of the Amazon is so large and complex, it is
difficult to understand the true nature of the effects of acidity and acid
deposition. From the data collected thus
far, there seems to be a relationship between changes in the acidity of water
and pollution. Further research is vital
to the understanding of this relationship.
B. Mining
Mining has contributed to the amount of mercury found in the
Amazons rivers. It is estimated that
2000 tons of mercury have been dumped into the Amazons rivers over the past
century alone (Brown et al., 2002). It has been demonstrated that at times, the
rate of mercury production is equivalent to the rate of gold production (Veiga).
The processes currently employed by miners utilize mercury
to clean the gold. Often mercury is not properly disposed of, and instead is
subsequently passed on to nature for disposal.
Although mercury storied in the soil is in a harmless, organic form,
mercury in the water is converted to methyl-mercury, which is one of the most
poisonous substances known to man (Veiga).
Methyl-mercury filters down the river systems to communities
down stream of the mining sites. Studies have proven that villagers in these
areas suffer from the effects of mercury in their waters. Miners themselves
have been victims of mercury poisoning as well.
In summary, mining requires a new cleaning method, one that
either does not employ mercury at all, or makes clean up of the mercury used
more effective. The trouble here will be convincing miner to switch to a new
gold extraction method.
Another problem with mining is that it leaves large holes in
the earth. As a result, mining sites are often covered by stagnant pools of
water. Such pools are breeding grounds
for mosquitoes, an in particular, mosquitoes which carry malaria (Brown et al.,
2002). These mosquitoes are notably feared as they cause malaria in the local
populations. Recently, malaria has become a widespread epidemic in
Fortunately, malaria is a simple disease to
prevent. The simplest solution is to
remove the stagnant pools of water.
Miners should be required to cover any holes which are created during
the mining process. This simple method is not performed by the miners although
they are largely responsible for the increase in malaria cases in
To improve mining and decrease its negative
effects on the environment requires a fundamental change in the minds of
miners. Incentives could be awarded to miners whose mining sites have been
found to be compliant with certain standards established for environmental
protection.
Next: Hydroelectric Power -->