UNIFIED ENGINEERING Fall 2006
Ian A. Waitz
Problem T4 (Unified Thermodynamics)
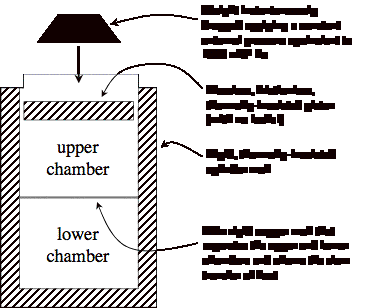
Below is a piston-cylinder
arrangement where the piston has two chambers. Although the cylinder is thermally-insulated from the
surroundings, the lower chamber is isolated by a thin copper barrier, which is
rigid but which allows the slow transfer of heat between the two chambers. The cylinder walls are
rigid. The chambers are filled
with air which behaves as an ideal gas with R=287 J/kg-K and you can assume the
specific heats are constant at cv=716.5
J/kg-K, cp=1003.5 J/kg-K.
Both chambers start in
thermodynamic equilibrium with T=300K, p=100x103 Pa with a volume of
0.1 m3. A weight
equivalent to an external pressure of 1000x103 Pa is instantaneously
dropped on the upper cylinder. You can assume that the piston itself is
massless and free to move without friction. Your objective is to devise a simplified thermodynamic
model of this system and to use it to estimate the temperature the gas in the
lower chamber would come to when the two-chamber system eventually comes to
thermodynamic equilibrium.
a)
Describe the energy exchange processes in the device
in terms of heat, work and various forms of energy. (LOÕs #1, #2)
b)
What processes will you use to model this system? Why? (LOÕs #2, #4, #5)
c)
What is the temperature the gas in the upper chamber
comes to shortly after the instantaneous dropping of the weight? (LO #4)
d)
What is the temperature the gas in the lower chamber
comes to when the whole system eventually reaches thermodynamic equilibrium?
(LO #4)