- There are many
different power and propulsion cycles, and we have only looked at a
few of these. Many other cycles have been devised in the search for
ways to increase efficiency and power in practical devices.
- We
can view a given cycle in terms of elementary Carnot cycles, as
sketched in Figure 6.5. This
shows that the efficiency of any other cycle operating between two
given temperatures will be less than that of a Carnot cycle.
- If we view the thermal efficiency as
(derived in
Section 8.5), this means that we should
accept heat at a high temperature and reject it at a low temperature
for high efficiency. This objective must be tempered by
considerations of practical application.
- The cycle diagrams in
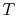 -
-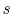 and
and 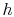 -
-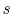 coordinates will only be similar if the working
medium is an ideal gas. For other media (for example, a two-phase
mixture) they will look different.
coordinates will only be similar if the working
medium is an ideal gas. For other media (for example, a two-phase
mixture) they will look different.
- Combined cycles make use of
the rejected heat from a ``topping'' cycle as heat source for a
``bottoming'' cycle. The overall efficiency is higher than the
efficiency of either cycle.
UnifiedTP
|

