Our studies on the cloning, recombinant expression and biochemical characterization of heparinases I, II and III represented the first detailed investigation of the catalytic mechanisms of
HSGAG-degrading enzymes
(Sasisekharan, et al., 1996, Ernst, et al., 1996, Godavarti, et al., 1996).
Through a combination of chemical modification, proteolytic mapping, and site-directed mutagenesis experiments, our laboratory has identified specific residues that are critical for the enzymatic activity of the individual heparinases.
Recently we identified Cys135, His203 and Lys199 as putative active site residues in heparinase I
(Sasisekharan et al., 1995, Sasisekharan 1996, Godavarti, et al., 1996, Godavarti, et al., 1998).
The calcium binding sites in heparinase I were also mapped and characterized (Shriver, et al., 1999, Liu, et al., 1999).
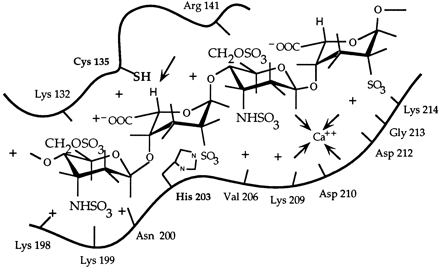 Schematic representation of the active site of heparinase I. The figure shows a heparin tetrasaccharide (about 0.165 nm in length;Nieduszynski(1985)) bound to the 18-amino acid heparin binding site. It is interesting to note that several secondary structure prediction algorithms suggest that the 18-amino acid heparin binding site contained the main alpha-helix forming potential in heparinase I, which is otherwise dominated by beta-sheet forming potential (Sasisekharan, 1991; Yoder et al., 1993a). It is hypothesized that the core fold of heparinase I is dominated by beta-sheets (as seen in pectate lyase; Yoder et al., 1993a) and that the region around 195-221 forms a large loop region with perhaps a partial alpha-helical secondary structure. Cysteine 135 and histidine 203 are in close proximity to each other and to the hydrogen (of the iduronate) marked by an arrow. Flanking these residues (cysteine 135 and histidine 203) are the heparin binding consensus sequences (lysine 198 to aspartic acid 204 and glutamic acid 207 to aspartic acid 212), the calcium co-ordinating motif (valine 206 to glycine 213), and lysine 132 and arginine 141 which together form the heparin binding domain of heparinase I. 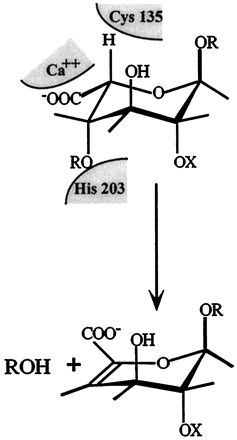 Schematic model of the catalytic domain of heparinase I. Cys135, His203, and Lys199 were identified as catalytically critical residues in previous studies (9, 11, 12). We propose that Ca2+ functions as a Lewis acid acting on the carboxyl group. Lys199 could act as an acid catalyst to protonate the carbonyl oxygen in the carboxyl group. Ca2+ could also act to stabilize either deprotonated Lys199 or Cys135. The polarization of the carboxyl group by Ca2+ or lysine would lower the pKa of the -proton at C-5 and facilitate the abstraction by cysteine of heparinase I. His203 would act as a second acid catalyst to protonate the leaving beta-substituent. In addition, we have identified three histidines, His238, His451, and His579 that are important for the enzymatic activity of heparinase II (Shriver, et al., 1998). Finally, in heparinase II we have identified a cysteine, Cys348, which is important in the breakdown of heparin-like regions by heparinase II but not heparan sulfate-like regions (Shriver, et al., 1998). With this mutant, we have generated a mutant enzyme with novel substrate specificity. Heparinase III is a unique member of the bacterially derived heparinase family. First, it is the only member that exclusively cleaves heparan sulfate-like regions of an HSGAG chain. Also, heparinase III contains no cysteine residues in its primary amino acid sequence. As mentioned above, biochemical studies of heparinase I and II have shown that cysteine plays an active role in the eliminative cleavage of the HSGAG backbone. Heparinase III does, however, contain twelve histidines (Godavarti, et al., 1996). Preliminary biochemical and site-directed mutagenesis studies have identified a critical histidine residue that is necessary for the catalytic degradation of heparan sulfate by heparinase III. Future studies in our laboratory will examine other amino acids that are involved in the active site chemistry of heparinase III with the goal of understanding the complete enzymatic mechanism responsible for the depolymerization of heparan sulfate. We also are currently attempting to clone the other HSGAG-degrading enzymes to increase the number of tools in hand to study HSGAG structure-function relationships. Several analytical techniques, along with our enzymatic tools, have proven useful in studying the heparinases at the molecular level. In collaboration with Prof. Biemann's laboratory Department of Chemistry, MIT, we have begun to develop a methodology analyzing HSGAG fragments generated upon heparinase treatment using capillary electrophoresis (CE) and mass spectrometry (MALDI-MS) (Rhomberg, et al., 1998, Ernst, et al., 1998). We have recently used this methodology for defined oligosaccharide substrates to identify the saccharide degradation products of heparinase I (and heparinase II) in order to understand how heparinase I (and heparinase II) cleaves its HSGAG substrate (Rhomberg, et al., 1998). 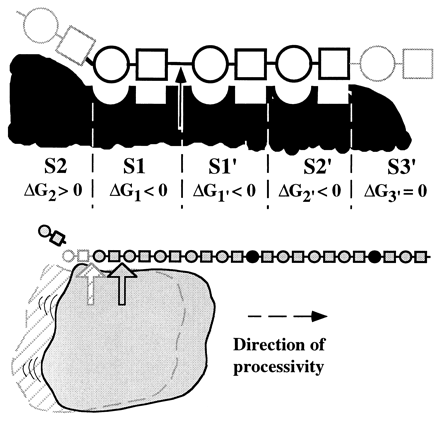 This novel approach is the first important step in the development of a practical HSGAG sequencing methodology. We are currently using the CE and MALDI-MS methodology to examine the role that Ca2+ might play in the processivity of heparinase I (Liu, et al., 1999). Our laboratory has also developed and optimized procedures whereby the different saccharide products of heparinase action on HSGAGs can be isolated in pure, large quantities. These HSGAG isolation techniques help provide us with a comprehensive pool of substrates with which to study the heparinases at the molecular level. To date, our research has given us an in-depth understanding of the molecular events associated with the enzymatic depolymerization of HSGAGs by the heparinases. We are continuing biochemical and structural studies of the heparinases to provide the necessary framework for us to realize two goals. First, an increased understanding of the action of the heparinases will allow for the design of mutant enzymes with altered substrate specificity, increased stability, and less variable activity for use in biochemical research and clinical applications. Second, our continuing research will facilitate the use of the heparinases as tools to elucidate structure-function relationships of HSGAGs. |
