There are two general approaches to the study of human aging. One approach involves studying the diseases associated with old age. This includes diseases such as alzheimers disease, arthritis, cancer and heart disease. Since the majority of people die from these types of diseases, it should be possible to extend the average human life span by developing treatments.
The other approach to reaseach on human aging is to study the aging process itself. This is more analogous to the types of research done on model organisms such as C. elegans and S. cerevisiae. If the genetic and biochemical causes of aging can be identified, then it should be possibel to intervene in these processes to extend both the mean and maximum life span of our species. Work in the Guarente Lab is focused on this approach to aging research. Currently, there are two projects in the lab that specifically address aging in humans.
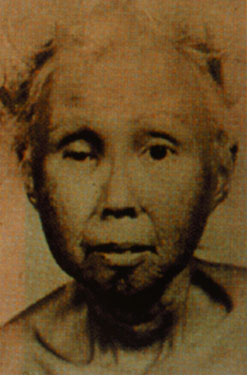
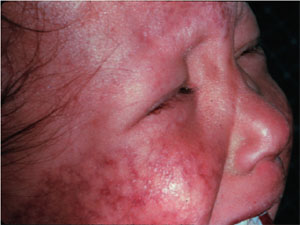
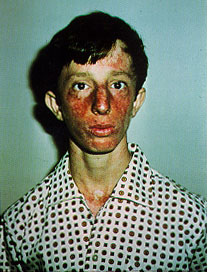
We are interested in studying the human RecQ homologs, in particular the WRN protein. Mutation of WRN results in the progeroid disease Werner syndrome. Werner syndrome patients exhibit many symptoms associated with premature aging including hair loss, graying hair, osteoporosis, and cataracts. Two other human diseases, Rothmund-Thompson Syndrome and Bloom Syndrome, are also associated with mutation of RecQ homologs.
.
|
Werner
Syndrome
|
Rothmund-Thompson Syndrome |
Bloom
Syndrome
|
 |
 |
 |
|
Each disease is
characterized by genomic instability, cancer, and:
|
||
|
|
|
Two postdoctoral associates, Brad Johnson and Bob Marciniak, are currently examining the role of WRN in telomere maintenance. Cultured cells lacking WRN senesce more rapidly than wild type cells and this phenotype is suppressed by ectopic expression of telomerase. Recently, a Johnson et al. (2001) have reported a role for the only yeast RecQ-family member, SGS1, in telomere maintenance. The function of RecQ homologs in mice is also being studied.
Human SIR2
Based on our findings that overexpression of SIR2 extends life span in both yeast and worms, it is obviously a high priority to determine whether this conservation of function extends to mammals, and humans in particular. While it is trivial to answer this question in mice, we are unable to directly assess the situation for humans. One indirect approach we are taking is to look for SNPs, small nucleotide polymorphisms, that are tightly linked to the various human SIR2-family members.
The idea is relatively simple. For example, lets assume we find a SNP tightly linked to a SIR2 homolog, and there are two alleles of this SNP present at high frequency within the population. We can then ask whether, in a large number of people, one allele is found more often associated with people that live to be over 100 years old. If it can be demonstrated in a statistically significant manner that allele A is associated with long life span and allele B is associated with short life span, this suggests that the particular SIR2 homolog our SNP is linked to may regulate longevity. This work is being carried out by Brad Johnson in collaboration with the Framingham Heart Study group.
