Coiled coils
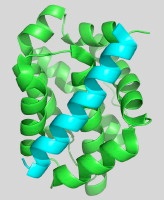
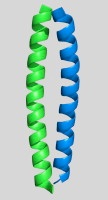
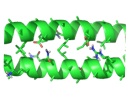
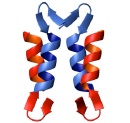
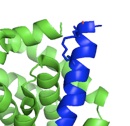
The alpha-helical coiled coil is an extremely common interaction motif found in proteins with many different biological functions. Coiled-coil structures consist of two or more helices that wrap around one another with a superhelical twist. Their sequences are characterized by a seven amino-acid repeat, (abcdefg)n. The a- and d-position residues are predominantly hydrophobic and the e- and g-position residues are often polar or charged. Their simple sequence patterns and symmetrical structures make coiled coils amenable to computational modeling. Short lengths and autonomous, reversible folding make some examples very experimentally tractable as well. A rich body of literature dating to the 1950s reports many sequence/structure/function relationships for coiled coils, as well as an abundance of important roles in biology. Areas of particular interest to the lab are highlighted below.
Interaction specificity
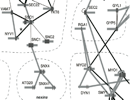
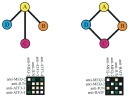
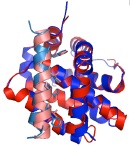
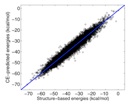
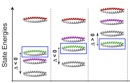
An important, unanswered question about coiled coils is how interaction specificity is encoded in sequence. Coiled-coil helices can form a variety of complexes of differing topology, including parallel and antiparallel dimers, trimers, tetramers, pentamers and even a reported heptamer. Also, coiled-coil-forming sequences are confronted with an enormous variety of potential partner helices in the cell. We are studying how the correct complexes are specified by amino-acid sequence, using both computational and experimental approaches. In recent years, we have focused on the interactions of bZIP transcription factor leucine zippers, which form parallel coiled-coil dimers.