
| Home |
| Administration |
| Research Areas |
| Core Facilities |
| UROP |
| Location |
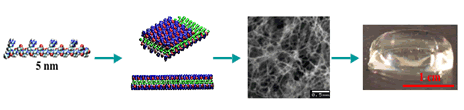
Cell and Tissue Engineering CBE researchers are forging new pathways in multi-group collaborations involving the use of stem cells and self-assembling peptide scaffolds for tissue engineering, the basis of a large ongoing Bioengineering Research Partnership (BRP) Grant from NIH centered on bone, myocardium, cartilage, bone, and liver tissue. This work is related in part to our industry connections with 3-D Matrix, Inc. (scaffold), Olympus (bone tissue engineering), and our work with Centocor (J&J) on cartilage degradation in osteoarthritis and the need for regenerative technologies. Close connections to Harvard Medical School, Colorado State University Medical School, the Cleveland Clinic, Rush University Medical School, and the University of Bern, Switzerland are central to ongoing research. |
|
|---|---|
 |
|
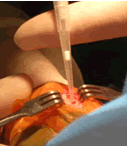
Exciting progress has been made in the use of peptide scaffolds in cartilage tissue engineering. A recently completed study in rabbits has now shown the ability of specially designed peptide gels to be used as an injectable, in vivo, and to provide a new material that can fill full-thickness osteochondral defects and promote improved cartilage repair. These scaffolds also deliver growth factors and marrow-derived stem cells to a joint lesion. A study using these peptides for repair of defects in the knees of horses has been initiated, enabling evaluation in a clinically relevant-sized defect with strenuous exercise, as would be the case in human. These studies involve collaborators in Clinical Sciences, Colorado State University and CBE faculty laboratories at MIT. |
 |
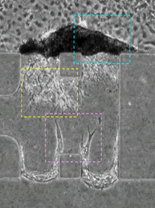
A collaboration between investigators spanning engineering, science and medicine has focused on the goal of creating a physiologically relevant microenvironment for liver morphogenesis and growth using self-assembling peptide gels with adhesive modifications and tethered epidermal growth factor (tEGF) under conditions of interstitial flow. Recent results showed that peptide biogels are promising substrates for the long-term culture of primary hepatocytes in a format such as the microfluidic system. In addition, new studies revealed that the seeding of hepatocytes under an interstitial “packing flow” (forcing the cells onto the surface of a 3D gel matrix) was successful in creating a liver-like mass of rat hepatocytes on one surface of the gel. These studies are the first published tri-culture system for studying the development of vascularized liver tissue. |
 |
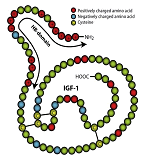
In collaboration with Brigham and Women’s Hospital scientists, CBE researchers have discovered a means for local delivery of insulin-like growth factor-I (IGF-1) with applications in cardiovascular and musculoskeletal pathologies. IGF-1 is a polypeptide protein hormone similar in molecular structure to insulin. It plays an important role in childhood growth and continues to have anabolic effects in adults as a stimulator of tissue growth and repair. However, systemic delivery of IGF-1 is not practical because of its short half-life in vivo and deleterious systemic side effects. A newly discovered fusion protein, HB-IGF-1, composed of IGF-1 with the positively charged heparin binding domain at the amino terminus of IGF-1, binds within tissues that contain a high density of negatively charged glycosaminoglycans (GAGs). This binding enables sequestration of HB-IGF-1 near cell targets in the tissue. Ipsen Pharmaceuticals is working with CBE scientists to provide GMP grade HB-IGF-1 to enable ongoing research aimed at translating this discovery into the clinic. |
 |
