Accurate laboratory results are imperative to understanding and solving the problem of arsenic-affected drinking water in Bangladesh; therefore, a major goal of this project was to determine accuracy and precision of the analytical chemistry results generated by various laboratories studying this problem.
The ICDDR,B laboratory performed exceptionally well during this study (see Table 1). The recoveries from the blind analysis of all independently prepared standards were within the 100 ± 25% range considered acceptable for routine analytical laboratories (9). An analytical interference for the determination of ferrous iron in undiluted groundwater by the 1,10-phenanthroline method was identified from suppressed matrix spike recovery (see Table 1).
| Analyte | Independent Standard Recovery |
Sample Matrix Spike Recovery |
|---|---|---|
| Arsenic (As) | 83% | 89 ± 11% |
| Ferrous iron (Fe2+) | 93 ± 10% | 34 ± 23% without dilution 96 ± 13% with 1 to 10 dilution |
| Total iron (Fe) | 95% | 120 ± 12% with 1 to 10 dilution |
| Sulfate (SO42-) | 106% | 106 ± 20% |
| Chloride (Cl-) | 114% | 90 ± 15% |
| Phosphate (PO43-) | 88% | 84 ± 2% |
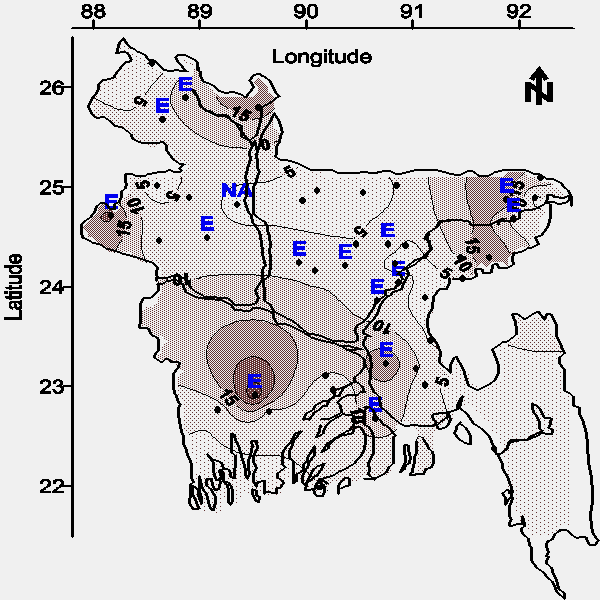
This interference was defeated by diluting all ferrous iron samples 10 times with distilled water before color development. Similarly, all samples submitted for total iron analysis by the 1,10-phenanthroline method were diluted 10 times with distilled water before acidic digestion to defeat this interference; despite this precaution, 24 of 89 samples (27%) failed to develop proper color. Some of these samples generated a whitish colloidal precipitate upon the addition of 1,10-phenanthroline. This analytical interference coupled with the results shown on Figure 10 (located on the map with the letter "E") suggests that one or more of the following potentially toxic metals are widely distributed in groundwater throughout Bangladesh: chromium, zinc, cobalt, nickel, bismuth, cadmium, mercury, and/or silver (5).
 |
| Figure 10. Map of the average total iron concentration (mg/L) in water from tubewells less than 30.5 m (100 feet) bgs. The distribution of analytical interference for the determination of iron is located on the map with the letter "E". |
The recovery of independent standards and sample matrix spikes were used to assess other laboratories in Bangladesh (see Table 2).
| Sample Description | Independent Laboratory |
Result (mg/L) |
|---|---|---|
| Standard solution = 1 mg As/L | Laboratory 1 | 4.891 |
| Distilled water = 0 mg As/L | " | 0.002 |
| Sample A (0.25 mg As/L) | " | 1.101 |
| Sample A (Blind duplicate) | " | 1.035 |
| Sample A (Blind triplicate) | " | 0.266 |
| Sample A plus 6.3 mg As/L | " | 24.126 |
| Sample B (0.31 mg As/L) | " | 1.109 |
| Standard solution = 1 mg As/L | Laboratory 2 | 0.533 |
| Sample B (0.31 mg As/L) | " | 0.397 |
| Sample B plus 3.3 mg As/L | " | 0.884 |
| Standard solution = 1 mg As/L | Laboratory 3 | < 0.5 |
| Sample B (0.31 mg As/L) | " | 0.30 |
| Sample B plus 7.1 mg As/L | " | > 1.0 |
The arsenic results from laboratory 1 are systematically high by approximately a factor of 4; therefore, a simple calibration error was likely generating results which incorrectly suggest that the arsenic problem is 4 times worse than reality. laboratories 2 and 3 only recovered approximately 50% of the arsenic from independent standards, such results underestimate the significance to the arsenic problem by a factor of 2.