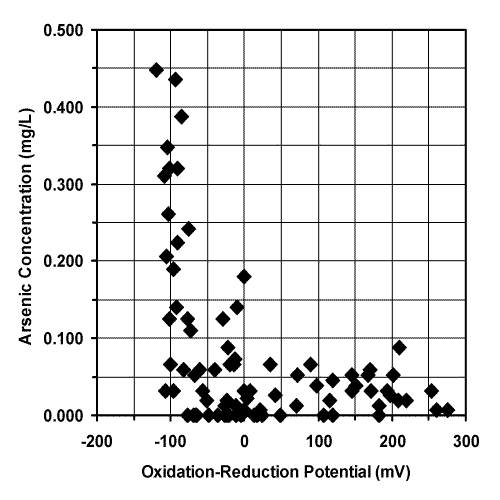

The graphs of arsenic concentration versus oxidation-reduction potential and arsenic concentration versus pH are shown in Figures 14 and 15, respectively.
 |
 |
The increase in arsenic concentration at relatively low oxidation-reduction potentials and moderate pH values suggests that soluble As(III), rather than difficultly soluble and potentially colloidal As(V), was the dominate form in the most highly affected tubewells (19). If the arsenic is in the soluble As(III) oxidation state, then oxidation to difficultly soluble As(V) followed by coagulation, filtration, or sorption is required for effective treatment (16).