
Aluminum Components for Automobiles:
Casting Low-Cost, High-Quality Parts
Despite those and other advantages, semisolid processing did not take off until the mid-1990s, when the automotive industry began calling for parts that were lightweight, low-cost, reliable, and long-lived. Cast or forged components made of iron or steel began being replaced by components of aluminum alloys manufactured by semisolid processing. The advantages of semisolid processing over traditional methods have proved remarkable. Castings of semisolid rather than molten metal form quickly and have lower cooling requirements. Semisolids can be handled by robotic arms and yet still flow well enough to fill intricate molds--in fact, better than molten metals can. Less gas is entrapped, fewer voids form, the surface finish is smoother, and less shrinkage occurs. The parts produced have more consistent and better mechanical properties and closer tolerances; and they need little if any additional shaping after they are formed, saving manufacturing time and expense.
Each year, companies in the United States, Japan, and Europe now produce or use millions of parts made by semisolid processing, and the number is growing quickly. However, as often happens, the commercial adoption of the new process has far outpaced the development of a detailed understanding of how it works. For example, how do the length of stirring, the rate of cooling, and other processing conditions affect the microstructure of the semisolid material? How does that microstructure affect how the material flows? And how does that flow behavior affect the products formed? An Energy Laboratory team including Professor Flemings, Anacleto de Figueredo, and Yusuf Sumartha has been performing rigorous experiments to answer such questions. While the focus is on expanding fundamental understanding, the results will have practical implications, providing manufacturers better control over the process and the products it yields.
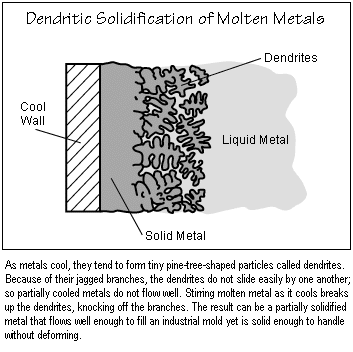
To understand why stirring changes the behavior of semisolids, one must
consider the microstructure of solidifying metals. Nearly all metals and
alloys of commercial importance solidify "dendritically." As the liquid
begins to solidify, tiny pine-tree-shaped particles called dendrites form
(see the figure below). These jagged particles do not slide by one another
easily, so the semisolid material does not flow well. Stirring the liquid
as it solidifies breaks up the dendrites as they form, tearing their branches
off. The particles are smoother and can slide by each other. As a result,
the semisolid flows more easily, even when its solid content is relatively
high. Professor Flemings likens the process to making ice cream. If you
combine all the ingredients for ice cream and place the mixture in a freezer,
the material formed will contain big ice crystals--not a very palatable
product. But if you stir the mixture while it is freezing, you break up
the ice crystals as they form. The ice cream remains smooth and creamy,
even when as much as 70% of it is solid.
Previous studies have looked at the flow behavior of semisolids, but their approach typically has not accurately reflected the industrial situation. For example, the studies tended to involve small quantities of semisolid material. The bulk quantities used in a factory might behave quite differently. And they generally involved measuring the viscosity of the semisolids--a property that does not translate directly into how well a semisolid will flow into the little spaces inside an industrial mold.
Professor Flemings and his colleagues therefore use a different approach--one that involves both a larger amount of metal and a different method of quantifying flow behavior. They melt an ingot of an aluminum alloy in a crucible. They then cool the molten metal while stirring it at a controlled speed between 0 and 1000 rpm. When the metal is partially solidified, they use a partial vacuum to suck some of it up a silica tube and then measure how far it travels before it solidifies. That technique, specially adapted for use with semisolid materials, is a simple but effective means of establishing "fluidity," a foundry concept that describes the ability of a metal to flow before being stopped by solidification.
In the experiments, thermocouples placed in the metal directly measure its temperature. Videos taken at 40,000 frames per second track how fast the semisolid moves up the tube, and quantitative metallographic analysis shows the microstructure of the specimens collected in the tube. (Although the specimens are solid, their microstructure is similar to the material in semisolid state: solidification occurs so quickly in the tube that the microstructure has no time to change.) Based on all the experimental evidence, the researchers relate fluidity to the microstructure of the semisolid, and the microstructure to processing conditions such as cooling rate, cooling time, and "fraction solid" (the fraction of the semisolid that is in solid rather than liquid form).
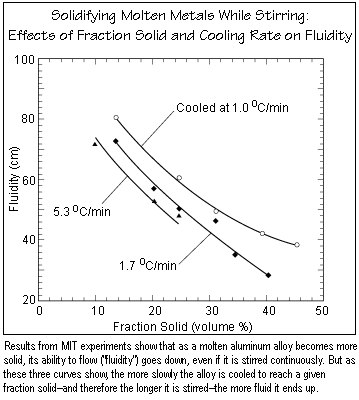
With so many interacting factors involved, identifying conditions that increase the fluidity of the semisolid is not straightforward. The figure below summarizes some of the experimental results. The curves show how fluidity changes as the fraction solid changes, with each curve representing a different cooling rate. Not surprisingly, as the semisolid becomes more and more solid, its fluidity decreases, regardless of the cooling rate. For a given fraction solid, a slower cooling rate produces higher fluidity. Thus, if the material is about 14% solid (by volume), cooling it at 1.7oC per minute produces a semisolid that travels about 73 cm up the tube, while cooling it at 1.0oC produces a semisolid that travels about 80 cm. The explanation for that trend is clear. If the molten metal is cooled more slowly, it will take longer for it to reach a given fraction solid. Assuming that stirring is continuous, the longer processing time translates into a longer stirring time, thus fewer dendrites and higher fluidity. (Stirring the solidifying ice cream longer makes it more creamy.) Videos taken with the high-speed camera show that even at a high fraction solid, the well-stirred semisolid material moves up the experimental tube smoothly and consistently.
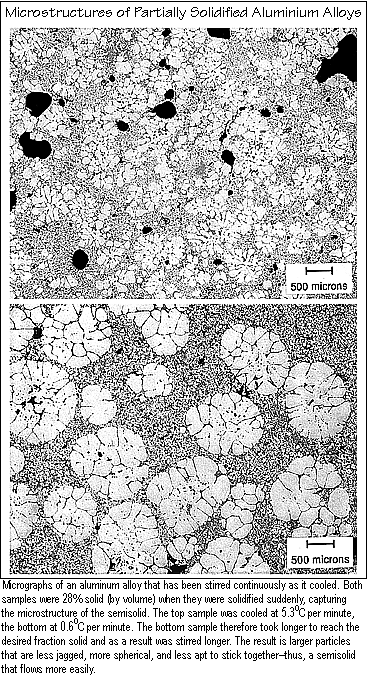
Almost more interesting are the microstructures that occur under the different processing conditions. For example, the figure below shows the microstructures of two specimens collected at the same fraction solid. The bottom one was cooled more slowly, thus stirred longer, than the top one. The lower cooling rate and longer stirring time produce larger particles. Yet the fluidity tests show that longer stirring increases fluidity. Several factors explain that seeming contradiction. For one thing, the larger particles are spherical, while the smaller ones are irregular in shape, thus less able to slide by one another. In addition, the smaller particles have a higher surface area and are more likely to interact and stick together than the larger particles are. The result is irregular agglomerates that move less easily than the large spherical particles do.
In those experiments, the researchers completely melted the aluminum
alloy and then worked with the semisolid as it formed during cooling. But
that approach replicates only one procedure used by industry. Parts manufacturers
may heat solid ingots made from stirred liquids to produce semisolid materials
to cast. To explore that situation, the researchers performed experiments
following the same procedure. They heated solid ingots specially prepared
by the Alumax Company until they were partly solid and partly liquid. They
then stirred the mixture for variable periods of time before sucking samples
up into the experimental tube.
The results are quite different from those of the tests starting with
molten metals. The researchers were able to make a semisolid shape that
was solid enough to be handled without deforming but soft enough to form
in a mold. However, for a given fraction solid, the specimens collected
in the experimental tube have substantially lower fluidity than do those
produced directly from liquids. Stirring the reheated semisolids makes
them flow more freely, but even after a full hour of stirring they are
not as fluid as the materials produced by stirring molten metal continuously
as it cools. Micrographs show that the original ingots contain relatively
fine, rounded particles. On remelting, those particles tend to form
three-dimensional, entangled strings. The strings are still present after
an hour's stirring, and some of them are as long as the experimental tube
is wide.
Thus, starting with remelted solid ingots is not as good as starting with fully molten metal. However, the drawbacks of using remelted solids are easily offset by the convenience and cost savings of not having to deal with large quantities of molten metal. While homemade ice cream may have a nicer taste and consistency than store-bought, most people choose to buy their ice cream in the store and let it melt a bit so it becomes smooth and creamy.
Experimental equipment now being designed and built by James Yurko will
provide the research team with an even closer look at how semisolids flow.
Instead of sucking the stirred semisolid up a tube, the new experimental
apparatus will push it through a small hole, much like pushing toothpaste
out of a tube. As a result, the semisolid will move about ten times faster
than in the previous experiments, achieving velocities close to those that
prevail in important industrial processes such as die casting. In addition,
the new equipment will be highly instrumented. The direct measurements
of velocity, pressure, and other important characteristics will provide
the researchers with a much improved fundamental understanding of how semisolids
flow during industrial processing.