
April-June 2000 Issue
New Simulation Tool for Designing Cleaner Diesels

April-June 2000 Issue
New Simulation Tool for Designing Cleaner Diesels
![]() he fuel-efficient diesel engine may face a bleak future unless engineers can
dramatically reduce its particulate emissions to meet likely future regulations.
Low-cost solutions may emerge if engineers can rethink engine and fuels technologies
in tandem, tailoring the fuel properties to the engine design and vice versa.
To help them achieve that match, Energy Laboratory researchers are formulating
a simulation tool that will predict the effects of changes in both engine
design and fuel composition on emissions and efficiency. The simulation generates
equations that describe chemical reactions occurring throughout the combustion
chamber and links them to reflect the interdependency of chemical composition,
flows, and temperatures in adjacent regions. Representing all the molecular
species in a combustor would require thousands of chemical models, so the
simulation instead deals with "functional groups"--groups of atoms that always
act as a unit and are building blocks for many types of molecules. Most important,
the simulation uses "adaptive chemistry," a novel concept that involves using
the simplest possible chemical model to analyze a given region. Based on numerical
analysis, the simulation determines which species and reactions are important
in a region and which ones it can leave out. (For example, why examine reactions
involving fuel molecules in areas where no fuel remains?) This approach simplifies
the computational task without sacrificing accuracy. While much work remains,
the new simulation may one day reveal ways to fine-tune a variety of combustion
devices and their fuels for cleaner operation, perhaps without major financial
investment.
he fuel-efficient diesel engine may face a bleak future unless engineers can
dramatically reduce its particulate emissions to meet likely future regulations.
Low-cost solutions may emerge if engineers can rethink engine and fuels technologies
in tandem, tailoring the fuel properties to the engine design and vice versa.
To help them achieve that match, Energy Laboratory researchers are formulating
a simulation tool that will predict the effects of changes in both engine
design and fuel composition on emissions and efficiency. The simulation generates
equations that describe chemical reactions occurring throughout the combustion
chamber and links them to reflect the interdependency of chemical composition,
flows, and temperatures in adjacent regions. Representing all the molecular
species in a combustor would require thousands of chemical models, so the
simulation instead deals with "functional groups"--groups of atoms that always
act as a unit and are building blocks for many types of molecules. Most important,
the simulation uses "adaptive chemistry," a novel concept that involves using
the simplest possible chemical model to analyze a given region. Based on numerical
analysis, the simulation determines which species and reactions are important
in a region and which ones it can leave out. (For example, why examine reactions
involving fuel molecules in areas where no fuel remains?) This approach simplifies
the computational task without sacrificing accuracy. While much work remains,
the new simulation may one day reveal ways to fine-tune a variety of combustion
devices and their fuels for cleaner operation, perhaps without major financial
investment.
The diesel vehicle is one of our most promising transportation technologies. It offers high fuel economy and low emissions of carbon dioxide. But whether it can meet future regulatory limits on other emissions, notably particulates and nitrogen oxides, is questionable. Changing the chemical composition of diesel fuels will not dramatically reduce emissions, largely because modern engines are designed to burn diesel fuels that vary widely in composition. But burning a well-defined diesel fuel in a specially tailored engine could bring large reductions in emissions without sacrificing efficiency. However, to achieve those advances, engineers need a better fundamental understanding of combustion and of the interplay between engine design and fuel properties.
For the past three years, Professors William H. Green and Paul I. Barton have been developing a tool that will help designers of diesel engines--and related devices such as burners and furnaces--foresee how proposed changes in equipment design and fuel composition will together affect efficiency and emissions. Their goal is to formulate a computer simulation that, given a specific engine design, fuel composition, fuel-air ratio, and other starting conditions, can calculate what reactions occur, what products form, how those products then react and interact, and what emissions ultimately go out the tailpipe. Defining all the necessary equations by hand would be impossible. Combustion in engines typically involves thousands of molecules that are chemically reacting while being influenced by flowing fluids and changing temperatures and pressures. Therefore, the researchers are working to make their computer simulation automatically perform as much of the job as possible.
Already they have made significant strides toward a better simulation tool than those now available. One big advance is a new computer algorithm that can construct a "reaction mechanism," a list of equations that describe the behavior of a given material under well-defined operating conditions. To compute the many rate parameters and physical property values required, the algorithm deals not with individual molecular species but rather with different types of functional atomic groups that tend to behave as a unit. This approach has several advantages. There are fewer distinct functional groups than molecules inside a combustor, and many of the functional groups prevalent in fuel molecules do not chemically react with one another. Some functional groups act as "conductors," meaning that electrons can move into and out of the group. If two conducting groups are next to each other and one changes chemically, the other will be affected. Thus, the simulation must treat those two functional groups together, first calculating the reactions of the individual functional groups and then calculating the subsequent interactions that occur between the two.
In hydrocarbon fuels, however, many of the functional groups are simple combinations of hydrogen and carbon that instead act as "insulators." Electrons do not pass into and out of them. As a result, groups separated by insulators can be treated separately. The presence of many insulating groups in hydrocarbon fuels thus simplifies the modeling task.
To perform an analysis, the simulation tool uses a library of reaction rules to define mechanisms for specific functional groups under specific conditions. With each mechanism, the simulation provides the appropriate rate constant (how quickly the starting material disappears) and the relevant thermochemistry (heat consumed or produced by the reaction). Rate constants for some reactions have been measured directly. In other cases, the simulation must estimate the rate constant using quantum chemical calculations and other advanced theoretical methods. The results are added to the library of information accessible by the simulation.
But conditions are not the same everywhere inside an operating engine. In some regions, cold fuel is entering; in others, hot fuel is burning; in others, soot is forming. The chemical composition and the temperature and pressure therefore differ at different locations and different times. And when conditions are different, even the same materials may react in different ways. One set of reactions may be important at high temperatures, a different set at low temperatures.
Therefore, the researchers couple their chemical simulation with an analysis of how air and fuel move and how conditions vary from place to place as ignition occurs and burning progresses. They use an approach called finite element modeling. They subdivide the entire combustion chamber into tiny cubes, each with its own local temperature, pressure, flow patterns, and species concentrations. Based on starting information about the fuel and air mixture and operating conditions, the simulation calculates all reactions of every species at every time step within every cube inside the engine. Moreover, the calculations for various cubes are linked, as the starting composition and conditions in one cube depend on the outcome of processes that have just occurred in neighboring cubes.
Not surprisingly, in most realistic situations this approach turns out to be computationally unmanageable. Simulating one combustion event (from ignition to burnout) requires analyzing the reactions and interactions of a thousand species within several thousand cubes at a million time steps--a job that would make even a supercomputer crash. One can make some progress by adjusting the number of time steps and the number of cubes, but one hits a limit. The chemistry happens so fast that one cannot use longer time steps or larger cubes and still get reasonable accuracy. Leaving out certain species or reactions is a possibility. Most simulations analyze processes in every cube using a single large chemical model that can handle all species and all conditions. Some modelers reduce the computation time by leaving out reactions they assume to be slow or species that appear sparse or of little practical concern. But such omissions can be risky. Under certain conditions those deleted reactions or species may be important.
Professors Green and Barton reduce the computational overload by taking a novel approach: rather than using the same model to analyze all cubes, they use different models in different cubes. For example, where ignition is occurring, the chemistry is very complicated. The models for cubes in that region therefore contain many species and reactions. Where fuel is burning, the chemistry is simpler; fewer species and reactions need to be monitored. And in cubes where exhaust is forming, the models need not try to track fuel molecules because there are none.
Their approach, called adaptive chemistry, involves simply deleting
reactions and species in cubes where prevailing conditions make those reactions
and species negligible. And rather than do this adapting by hand or based on
assumptions, they have the simulation determine numerically which species are
important and which are not within different cells. The diagram below shows
that process for a single cube in a single time step. A is a chemical species
that is involved in some reaction that produces B. The simulation calculates
a rate constant for that reaction at the prevailing temperature and pressure.
Since that rate is high, B can form quickly; and the simulation includes it
in the model. A is also involved in a reaction that forms the species X. Following
the same procedure, the simulation determines that X forms at a low rate, so
it excludes X from the model (assuming that it finds no other reactions forming
X at an appreciable rate). The simulation next examines the reaction by which
B forms Y. Again the rate is low, so Y is also excluded from the model. If in
some other cube conditions are different and one of the excluded species forms
at a high rate, the simulation recalculates the entire system with that species
included.
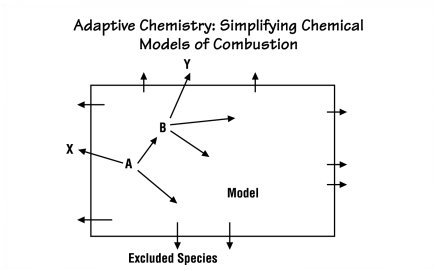 |
| MIT researchers are developing a simulation tool for analyzing chemical reactions inside a diesel engine. They simplify this computational challenge by using a method they call adaptive chemistry. Rather than using the same large chemical model to analyze all regions inside the combustion chamber, they use different models in different regions. This figure demonstrates how the simulation determines which species and reactions can be excluded at a given location and time. Here, a reaction involving species A makes species B at a rapid rate, so B is included in the model for this region. However, the rates at which A makes X and B makes Y are low, so both X and Y are excluded from the model. Leaving out "negligible" reactions and species reduces the needed calculations without sacrificing overall accuracy. |
Developing a workable system of models may take several hundred iterations, but the researchers have proved mathematically that there always will be a final solution because the list of species and reactions will never be infinite. In the end, each cell has its own chemical model that includes only the species and reactions that are important for those conditions at that time and place. Unnecessary computations are thus avoided whenever possible without loss of accuracy.
The researchers are continuing to develop and refine various aspects of their simulation to make it more accurate as well as more efficient. They are also calculating and measuring rate constants for more reactions and adding them to the simulation library. They are working to incorporate flow-modeling techniques that will better reflect the turbulence typical of combustion systems. And they are considering the challenges involved in working with more complicated fuels. Thus far, their work has focused on diesel fuel made from natural gas using the Fischer-Tropsch process. This pure, highly paraffinic fuel is simple to model and promises low emissions if matched with an appropriately designed engine. The simulation task will be harder with standard diesel fuel, which is far more variable and contains a huge number of different species. Another challenge will be tracking all of the intermediate species involved in the growth of giant soot particles. The ability to model particulate growth is important because particle size distribution may directly affect the potential health impacts of emissions.
Future regulatory limits on particulate emissions from diesels are not yet set. Epidemiological data suggest that particulates in diesel exhaust harm human health, but toxicologists cannot yet explain exactly how the damage occurs. As a result, there is much controversy about whether particulates from diesels are actually responsible for the observed health problems. Professor Green believes that confirming and clarifying the relationship between particulate emissions and health may take a long time. Meanwhile, engine designers may be able to ensure the future of the diesel by simply preventing particulates from forming. And having the ability to understand exactly how fuels and engines work together may point to cost-effective changes that could achieve that goal.