Please find work in 2004 on this alternate page.
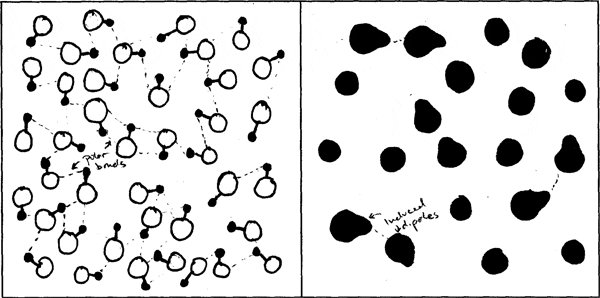
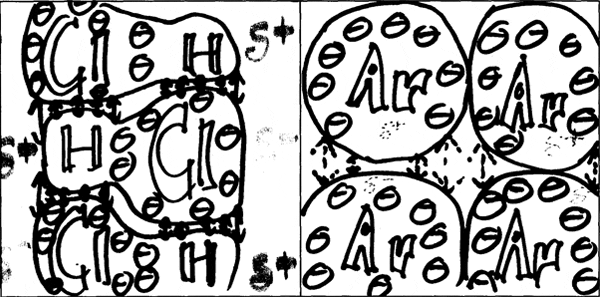
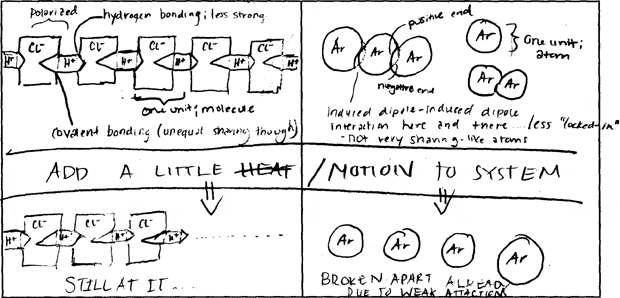
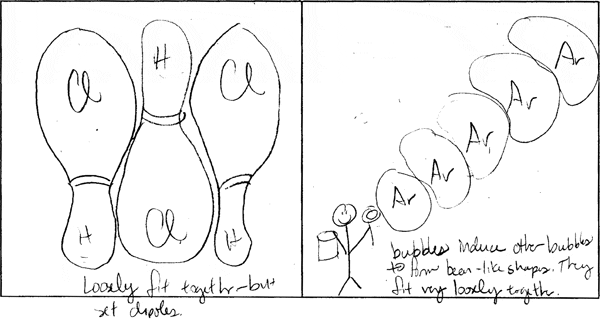
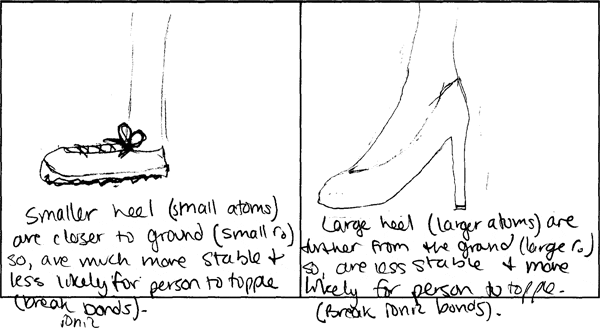
500 students in MIT Professor Donald Sadoway's Solid State Chemistry class were charged with making drawings to explain and compare molecular bonds in compounds with different boiling points. They were asked to consider physical features such as atom size and electron configuration to account for the forces at work within and between molecules, and which in turn determine the boiling point.
The following image pairs show very different ways of conceptualizing molecular bonding. All images by students from Solid State Chemistry, 3.091, fall 2005 at MIT.