|
|
||||||||
|
Chuang-Chung (Justin) Lee Department of Chemical Engineering, Massachusetts Institute of Technology Email: chchlee@alum.mit.edu Web: web.mit.edu/mcraegroup/ChuangChuang.html Tel: 781-577-6383 (O) Address: |
|
|||||||
|
Education: §
Ph.D. §
M.S. §
|
||||||||
|
Research Interests: § Kinetics of Amyloid Fibrillation |
||||||||
|
|
Claimed to be the number one cause of senile dementia,
Alzheimer’s disease is one of the disorders that involve misfolding of
amyloid protein and formation of insoluble fibrils. Although a variety of time dependent fibrillation
data in vitro are available, few mechanistic models have been developed. To bridge this gap we used chemical
engineering concepts |
|
|
|||||
|
from polymer dynamics, particle mechanics and population
balance models to develop a mathematical formulation of amyloid growth
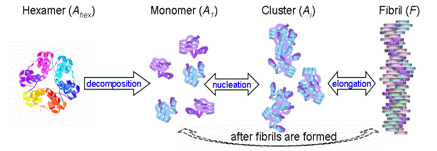
dynamics. A three-stage mechanism
consisting of natural protein misfolding, nucleation, and fibril elongation
phases was proposed to capture the features of homogeneous fibrillation
responses. In addition, the proposed
mechanism reflects the effect of each factor on fibril formation kinetics
such as protein types, initial concentration, seeding, and agitation over a
series of experimental conditions.
While our cooperative laboratory provided us with experimental
findings, we guided them with experimental design based on modeling
work. It was through the iterative
process that the size of fibril nuclei and concentration profiles of soluble
proteins were elucidated. The study
also reveals further experiments for diagnosing the evolution of amyloid coagulation
and probing desired properties of potential fibrillation inhibitors. |
||||||||
|
§ Short-term Plasticity |
||||||||
|
|
Depending on the types, synapses tend to depress or
facilitate under repetitive stimuli.
There were models and hypotheses describing individual reactions yet
few physiology-based models have been developed systematically to describe
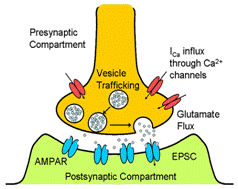
what and how key factors influence synaptic strength. In this paper, calcium dynamics at
presynapse were considered in describing the release of glutamates as
neurotransmitters; at postsynapse, binding of glutamate to AMPA receptor was
modeled as a first order delay system that outputs excitatory current. Since the model was kept mathematically
tractable, the analytical solution to the resonance frequency, which is the
most crucial determinant of plasticity tendency, could be derived. |
|
|
|||||
|
This unified model together with the closed form solution
is broadly supported by transient and frequency experimental data. Responses
from both depressing synapse and facilitating synapse can be explained by the
model. Based on the results of
parameter estimation, high initial release probability and low recovery rate
cause depression, which coincides with the vesicle depletion hypothesis. Furthermore, we were able to pinpoint the
parameters influential to the resonance frequency. High calcium initial concentration and gain
of calcium current to concentration result into high initial release
probability and thus lower resonance frequency. In contrast, for synapses less sensitive to
calcium or with higher recovery rate, high frequency stimuli are required to
get them saturated, so higher resonance frequency and thus facilitation are
observed. Based on the model,
experimental schemes were also suggested to switch the synapses from
depression to facilitation or vice versa. |
||||||||
|
§ Long-term Plasticity |
||||||||
|
|
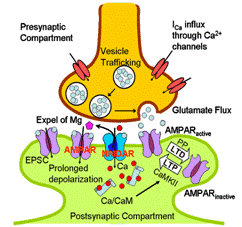
The accumulation of amyloid fibrils is suspected to cause abnormal modification of long-term synaptic plasticity which is viewed as the principal mechanism underlying learning and memory. Most synapses show long-term potentiation (LTP) or depression (LTD) which can last for more than hours after tetanus stimuli are applied and removed. Even though there are hypotheses explaining individual experimental findings, few systematic models have been built to specify the actual mechanism contributing to long lasting change. Therefore, we first considered vesicle trafficking in presynapse to describe the release of glutamate as neurotransmitter. Then in the postsynaptic compartment, we developed a calcium entrapment model to simulate the excitatory current and voltage. The systematic model consists of equivalent electrical circuits as well as ligand- and voltage-gated NMDA receptors. This built model is supported by a broad range |
|
|
|||||
|
of experimental measurements. According to the result of model
differentiation, we confirmed that calcium entrapment model explains graded
response of synaptic LTP better than biostability mechanism. In the mean time, we are beginning to
analyze new experimental data to assess the influence of amyloid fibrils on
the regulation of long term potentiation.
|
||||||||
|
|
||||||||
|
Publications: §
Journal Papers Lee, C.-C., Poon, C.-S., and McRae, G. J. (2009) “The Unified Theory of Spike Timing Dependent Plasticity”, J. Neurosci., in preparation. Lee, C.-C., Anton, M., Poon, C.-S., and McRae G. J. (2008) “The Unified Theory of Homosynaptic Short Term Depression and Facilitation”, J. Comut. Neurosci. In print, DOI 10.1007/s10827-008-0122-6. Abstract | Full Text Lee, C.-C.,
Nayak, A., Chien, W.-C., Lee,
C.-C., and Tai, C. Y. (2007) “Heterogeneous Nucleation Rate of Calcium
Carbonate Derived from Induction Period”, Ind.
Tai, C. Y., Chien, W.-C., Hsu, J.-P., and Lee, C.-C. (2001) “Supersaturation,
Induction Period, and Metastable Zone Width of Calcium Carbonate System”, Chem. § Conference Abstracts Nayak, A., Lee,
C.-C., McRae, G. J., and Sorci, M, Nayak, A., Lee,
C.-C., McRae, G. J., and Lee, C.-C.,
Nayak, A., Nayak, A., Dutta, A., Lee,
C.-C., McRae, G. J., and |
||||||||
|
Honors: Member of Biophysical Society, 2006-2007 MIT Class of 1936 Fellowship, 2003 Honorary member of of the Phi Tau Phi Scholastic Honor Society, 2001 Lee Yuan Tze Scholarship for Chemistry, 2000 Yen Family Scholarship,1998-1999 |
||||||||