MIT Experimental Petrology Laboratory
Department of Earth, Atmospheric & Planetary Sciences
Building & Room: 54-1220
Massachusetts Institute of Technology
Cambridge, MA 02139
E-mail: petrolab-www@mit.edu
Equipments in the MIT Experimental Petrology Laboratory
Equipment in our experimental petrology laboratory allows us to span a broad range of experimental conditions. The following photos show some of our instrumentation, facilities and activities that we undertake during the course of our experimental studies. These photos were taken in January 2008 and show many of the improvements that were possible through funding provided by the Instrumentation and Facilities program of the Division of Earth Sciences of the National Science Foundation (EAR-04472040).

Graduate student Jay Barr prepares a synthetic rock mixture for conditioning in a high temperature controlled oxygen fugacity gas mixing furnace. |

Mike Krawczynski and Jay Barr discuss mix preparation. |

Professor Tim Grove and graduate students Christy Till and Mike Krawczynski discuss petrographic features of a magmatic inclusion from Mt. Shasta volcano. |

Another view of our Olympus BX51 transmitted/reflected light polarizing microscope with digital camera and imaging software. This devive is an essential part of our operation. |

Graduate student Christy Till starts a high pressure – high temperature experiment on one of 3 Boyd-England piston cylinder devices in the lab. |

Professor Tim Grove assembling the piston cylinder device in preparation for an experiment. |

Jay Barr starting a high temperature – controlled oxygen fugacity experiment in one of 3 gas mixing furnaces in the laboratory. |

Jay Barr sealing a MHC (molybdenum hafnium carbide) cold seal pressure vessel used for 200 MPa H2O-saturated experiment on a basalt composition from Newberry Volcano, Oregon. |

Jay Barr monitoring experimental conditions of the MHC experiment. |
|

One-atmosphere gas mixing furnaces used for controlled oxygen fugacity experiments. In the foreground are holders for suspending the experimental charges in the hot spot of the furnace. Samples are suspended on thin noble metal wires that can be melted by an electrical current to provide for a rapid quench by dropping the sample into a dish of water (white bowl on table). |

Thin section of an experimentally produced mid-ocean ridge basalt (MORB) from a one-atmosphere controlled oxygen fugacity cooling rate experiment. Sample started as a spherical melt blob and grew crystals as the melt was cooled over its solidification interval. Sample is ~5 mm in diameter. Grey minerals are plagioclase. Red crystals are olivine and augite + pigeonite are intergrown with the plagioclase. Crossed polarized light. |
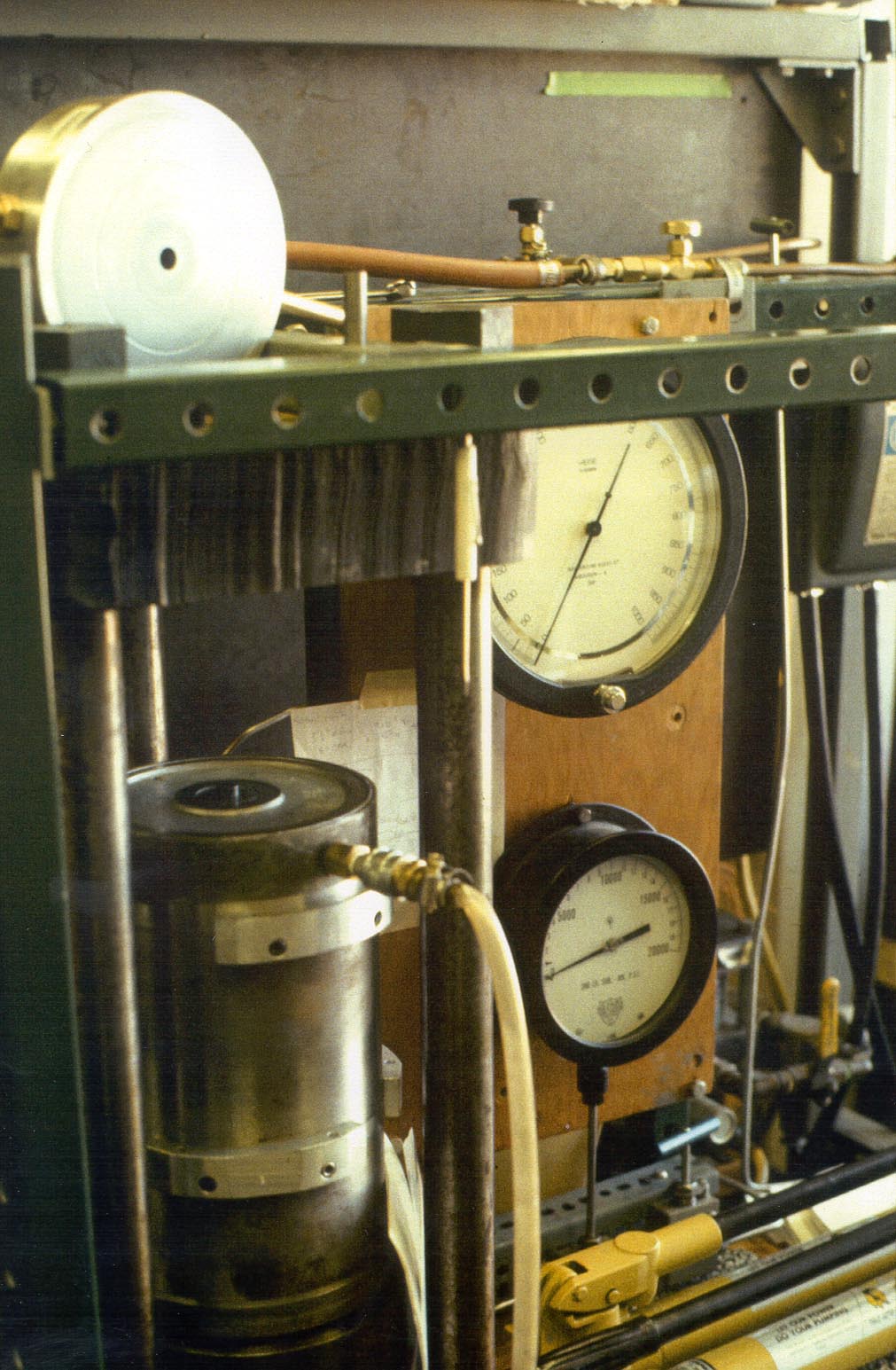
One of three piston cylinder presses used for experiments over the pressure range of 1 to 3 GPa. Press frame on the left holds two hydraulic cylinders. The one on the bottom provides an end load to keep the press together. A second "piston lad cylinder (inside the metal ring) applies a load to a 1/2" piston (visible in the center of the open space) that presses against a solid assembly that includes the sample, a graphite heater, and a pressure medium. The pressure vessel sits on the top of the press frame and the 1/2" hole for the pressure cell is visible. Gauges on the right are used to monitor loaf pressure that is applied with manual hand pumps. |
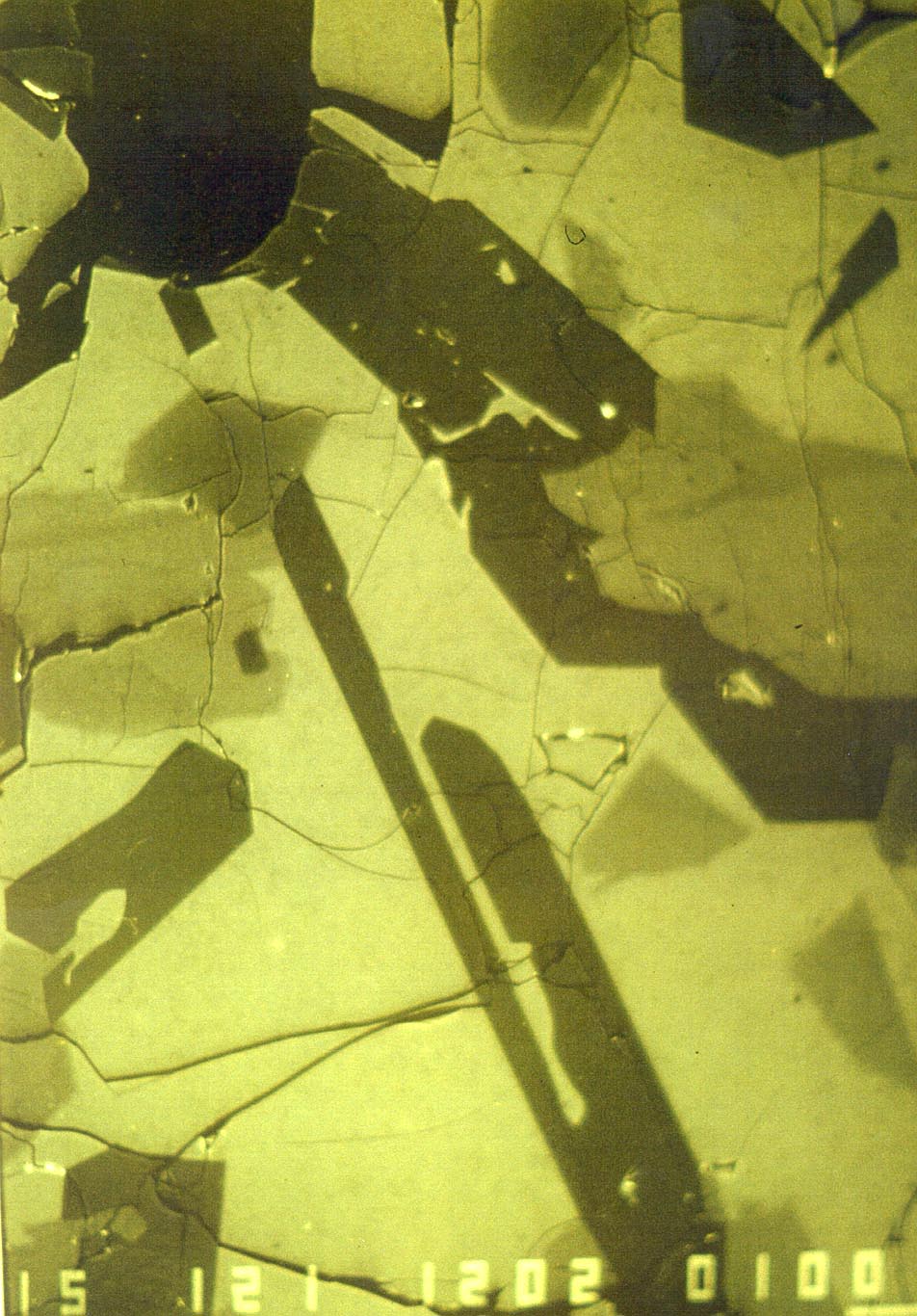
Backscattered electron scanning image of experiments H225 from Bartels et al. (1991) that contains augite (light gray), olivine (equant grey crystals, darker than augite), plagioclase (darkest gray skeletal crystals) and spinel (equant black crystal in upper right). Field of view is 0.5 mm. |

T.L. Grove quenching a TZM gas pressure vessel experiment. The pressure vessel is removed from the furnace while hot and inverted, allowing the sample to fall to the water-cooled upper pressure seal and rapidly quench. A gentle tap with a wrench encourages the sample to fall to the cooler part of the vessel (see Sisson and Grove, 1993; Bartels and Grove, 1991; Walker and Grove, 1993; Wagner et al., 1995; Grove et al., 1997). |

Examples of the signle crystal San Carlos olivine crucibles used in olivine-melt (Ehlers et al., 1991) paritioning studies and in sulfide liquid- sulfide melt partitioning studies (Gaetani and Grove, 1997). |
![]()
![]() E-mail: petrolab-www@mit.edu /
©2009-2012 Massachusetts Institute of Technology
E-mail: petrolab-www@mit.edu /
©2009-2012 Massachusetts Institute of Technology
 National Science Foundation
National Science Foundation