
Creating and Studying Ultra-cold Hydrogen
Our group has a long history of studying samples of ultra-cold, spin polarized hydrogen. Much of the pioneering leading to the ability of creating and trapping atomic hydrogen was developed in our laboratory. A crowning achievement of this work was the creation of Bose condensed hydrogen gas which was achieved in 1998.
Why Hydrogen
Atomic hydrogen has played a central role in the development and understanding of atomic physics. The simple structure of the hydrogen atom not only makes it valuable from a pedagogical standpoint, but also provides a system that can be theoretically described with remarkably high precision. In spite of the tremendous amount of theoretical and experimental attention given to the hydrogen atom, it has not yet divulged all of its secrets. Among other things, careful hydrogen spectroscopy can lead to more precise measurements of the Rydberg constant, and the development of highly stable atomic clocks.
Historic Experimental Techniques
Creating ultracold samples of atomic hydrogen offers a set of distinct experimental advantages over other methods of studying these atoms. Low temperature samples provide reduced inhomogeneous broadening effects, which in turn permit higher resolution spectroscopy. Magnet trapping, which is only possible at low temperatures, enables long observation times. The combination of magnetic trapping and low temperatures allows a great deal of control over the interactions between the hydrogen atoms and their environment. Unfortunately, these important advantages come at a high technical cost. The well documented experimental methods required for creating and studying ultracold hydrogen have been under continuous development for over twenty years. Although huge advances have been made, the required experimental techniques involve a complex synthesis of cryogenic and optical techniques. The cryogenic component of these techniques utilizes a dilution refrigerator for cooling the walls of an experimental cell to temperatures below 1K. Hydrogen atoms are disassociated in an RF discharge and thermalized with a superfluid 4He film that coats the inner walls of the cell. The cell resides in a superconducting magnetic trap which is deep enough to confine thermalized paramagnetic hydrogen atoms in their low-field-seeking spin states. Evaporative cooling is then used to cool the trapped samples into the ultra-cold regime.
In addition to the technical challenges, there are
fundamental physical constraints that not only limit the applicability of this
technique, but also limit the efficiency with which ultracold hydrogen can be
produced. The ability to load magnetic traps via thermalization to a superfluid
helium film is strictly limited to atomic hydrogen. Attempts with deuterium,
the only other feasible candidate, have shown that the D-4He binding
energy is too deep for the technique to be effective. Furthermore, the recombination
rate of deuterium on 4He surfaces is prohibitively large. For
hydrogen, the evaporative cooling process needed to enter the ultracold regime
just barely works. This is because the small H-H elastic scattering cross
section limits the thermalization process required for efficient evaporative
cooling.
Current Experimental Efforts
We are currently developing a new approach to creating ultracold hydrogen that promises to overcome many of the physical and technical shortcomings befalling the historic techniques for working with cold hydrogen atoms. Our new approach uses buffer-gas loading to introduce atomic hydrogen and lithium simultaneously into a magnetic trap. Evaporative cooling of the trapped mixture proceeds with much higher efficiency due to the large Li-H elastic scattering cross section. One of the more exciting features of this new approach is that it is not limited to H, and holds great promise for creating and studying samples of ultracold deuterium.
Buffer Gas Cooling
Figure 1 illustrates a buffer gas loading scheme that looks very promising for trapping 1 μB atomic hydrogen, lithium, and deuterium. A cryogenic cell is thermally anchored to the mixing chamber of a dilution refrigerator and placed within the bore of a superconducting, anti-helmholtz trapping magnet. The cell consists of two chambers separated by a mechanical valve. With the valve initially closed, ³He buffer gas is introduced into the lower chamber. See Figure 1.a. We focus a ~10 mJ, 5 ns pulse at 532 nm from a frequency doubled ND-YAG laser onto a solid sample of LiH. The YAG ablates the LiH producing a hot (~104 K) plume of gas containing both atomic H and Li. These hot atoms thermalize with the cold helium buffer gas and, after several microseconds, reach a temperature of a few Kelvin. Over tens of milliseconds this atom/buffer-gas mixture will cool further until the temperature of the cell walls (~350 mK) is reached. Throughout this thermalization process, the atoms will diffuse to the walls (where they stick) at a rate that depends on the strength of the magnetic trap. After the atoms are thermalized, the valve between the two chambers is opened. See Figure 1.b. A charcoal cryopump in the upper chamber quickly removes the buffer gas, leaving behind the trapped, thermally isolated atoms in the lower chamber. The trapped atoms are spectroscopically probed by passing a laser through the window at the bottom of the cell. The actual implementation of this buffer gas loading scheme is illustrated by the 3d rendered image of our cell shown in Figure 2.
Figure 1
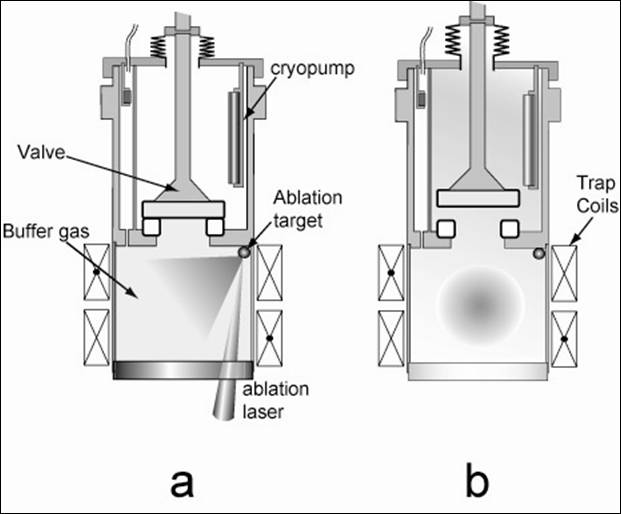
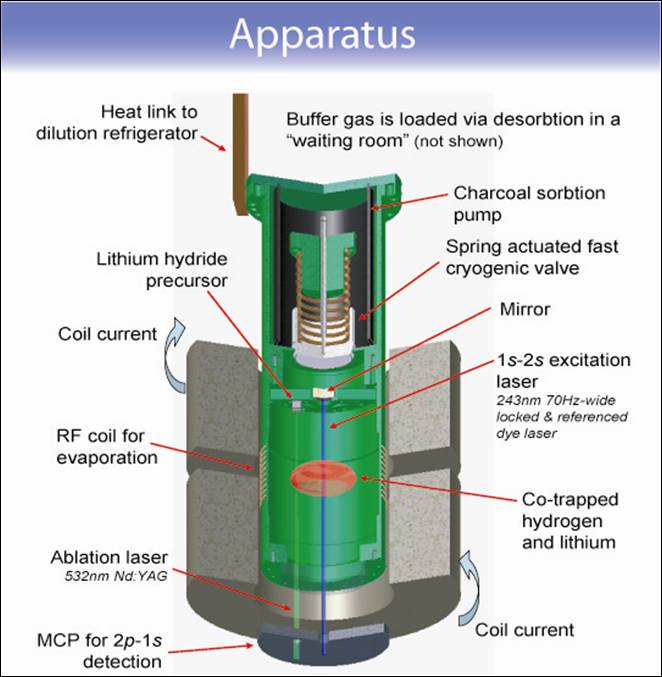
Figure 2


