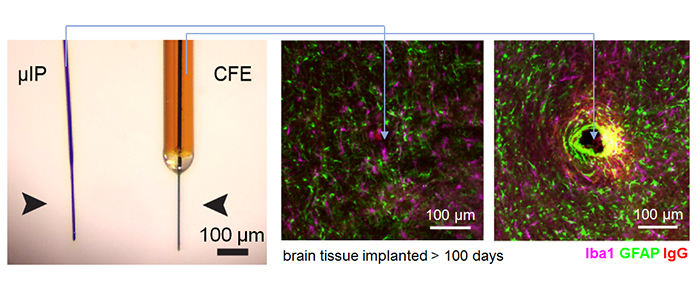
micro-invasive neural probes
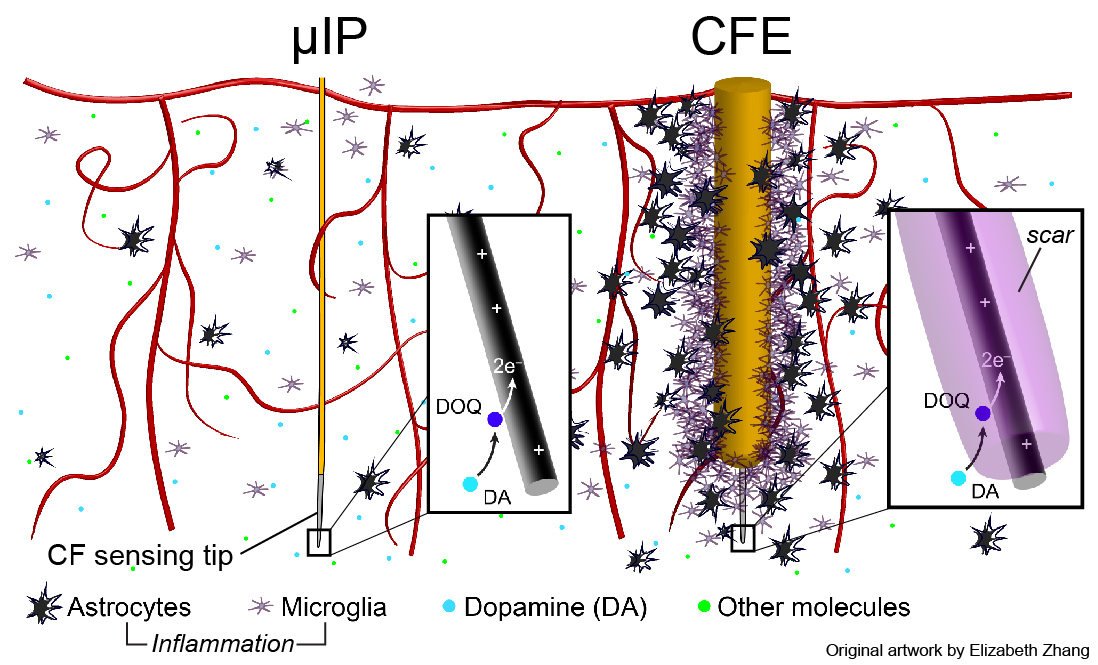
The brain triggers an inflammatory response (e.g. astrocytes, microglia, permeation of vasculature) to implanted devices [Kozai et al 2015], [Jaquins-Gerstl & Michael 2015]. This forms a scar that physically obstructs diffusion of targeted molecules to the sensor and causes permanent brain damage.
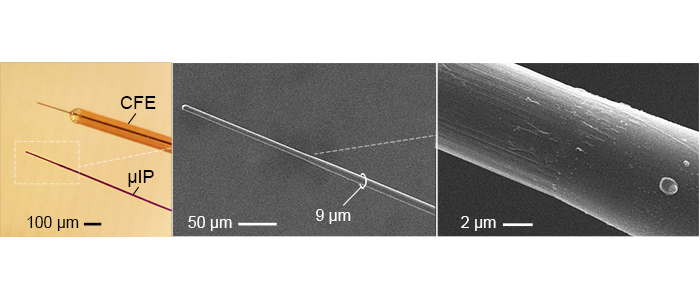
The micro-invasive probe (µIP) is ~the size of a neuron with a diameter < 10 µm, and 10 times smaller than state of art, CFE, neurochemical sensors. The reduced size reduced inflammation.
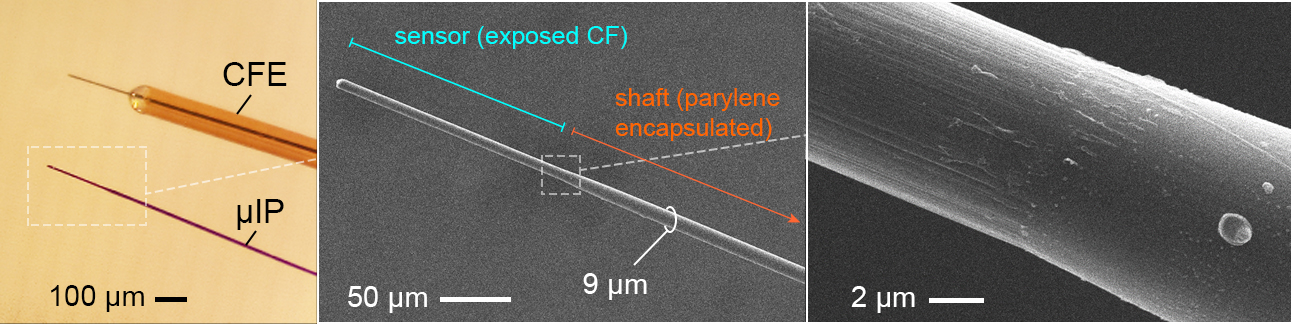
The µIPs are fabricated by encapsulating 7 µm diameter carbon fibers (CFs) with a conformal layer of parylene (<1 µm).
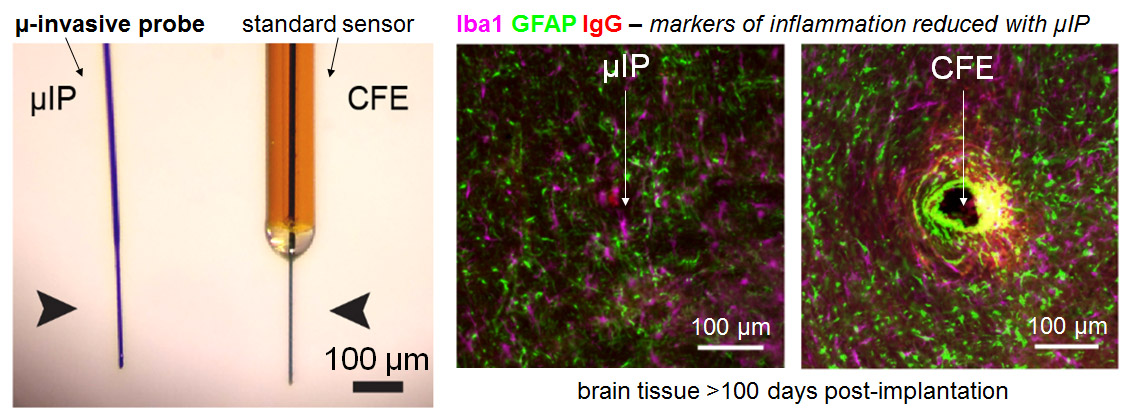
Inflammatory response is significantly reduced around the µIP in comparison to standard CFE sensors in terms of the expression of astrocytes (GFAP), microglia (Iba1), and blood brain barrier permeability (IgG). Very little difference was observed between the µIP implanted brain and unpenetrated brain.
references:
Schwerdt et al., Cellular scale probes enable stable chronic subsecond monitoring of dopamine neurochemicals, Comm Bio, 144, 2018.
Schwerdt et al., Subcellular probes for neurochemical recording from multiple brain sites, Lab Chip, 17(6), 2017.
chronically stable dopamine detection
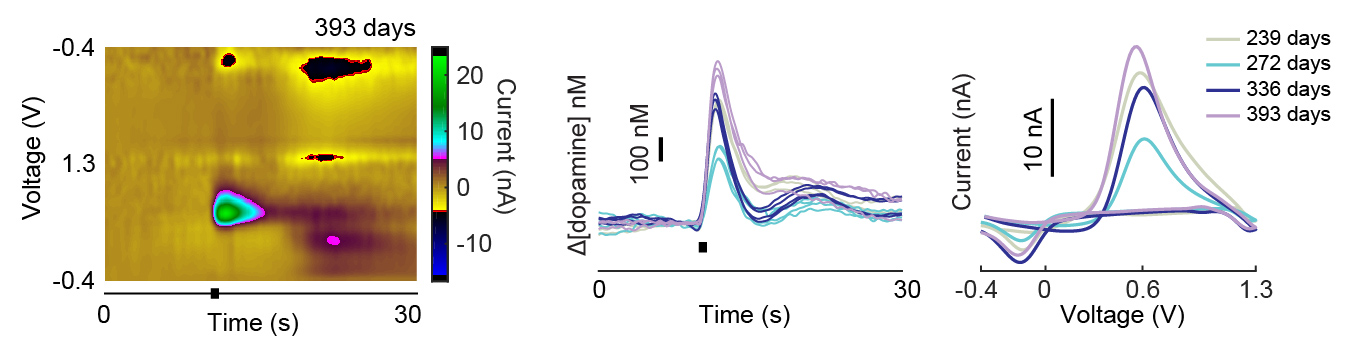
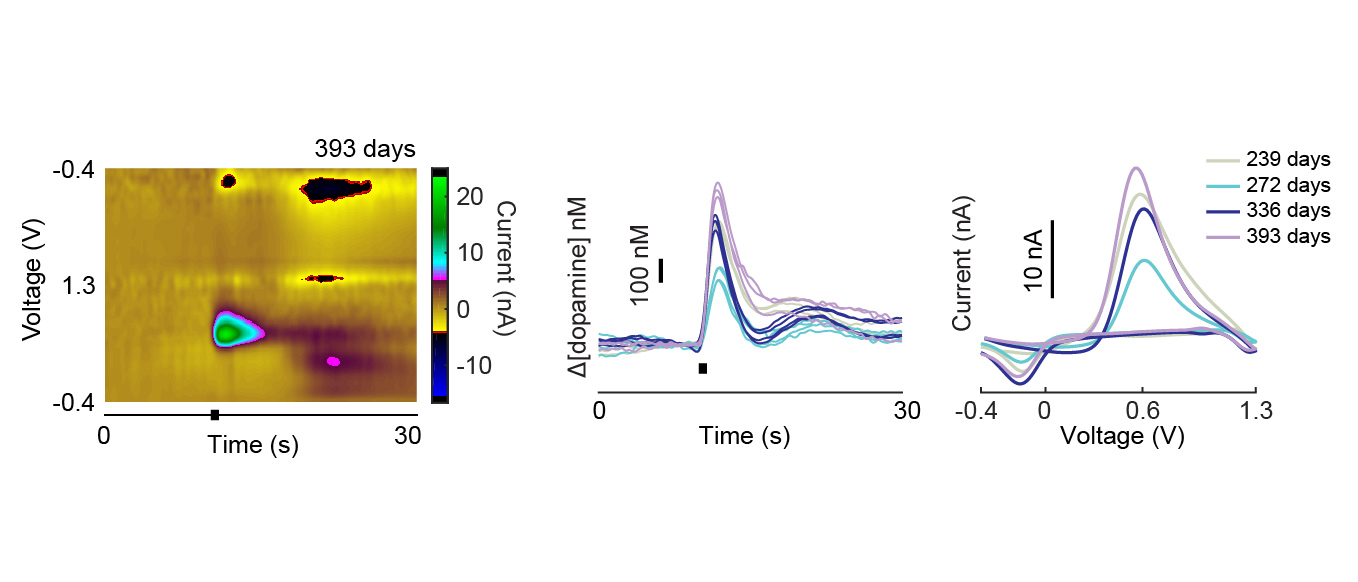
The ability of implanted probes to record dopamine neurochemicals has been shown to degrade over time (amplitudes and rate of detected signals decreases over time) [Schwerdt et al 2017].
The micro-invasive probe (µIP) provided longitudinally stable monitoring of dopamine (sensitivity was uniform across time and the recorded amplitudes did not show a significant trend over time).
references:
Schwerdt et al., Cellular scale probes enable stable chronic subsecond monitoring of dopamine neurochemicals, Comm Bio, 144, 2018.
dopamine monitoring in primates
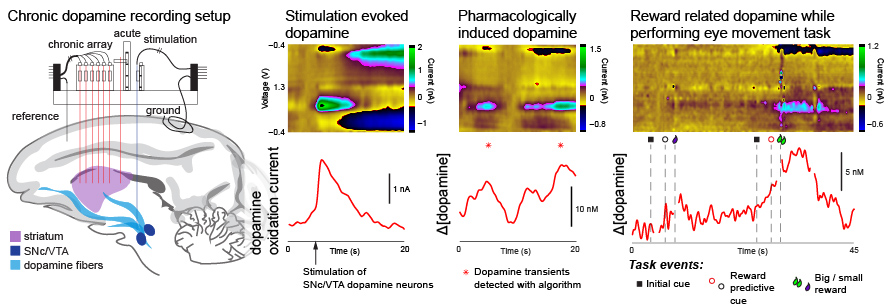
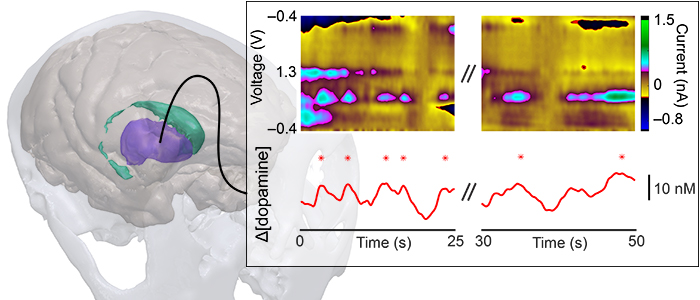
Sensors capable of chronic use in primates are essential to understand dopamine function in behavior and to provide viable diagnostic methods for detecting and targeting treatment for Parkinson's disease and other neurological disorders.
We developed systems capable of monitoring dopamine over several months in the nonhuman primates as a step towards facilitating translation to humans.
references:
Schwerdt et al., Long-term dopamine neurochemical monitoring in primates, PNAS, 114(50), 2017.
arrayed systems for large-scale mapping
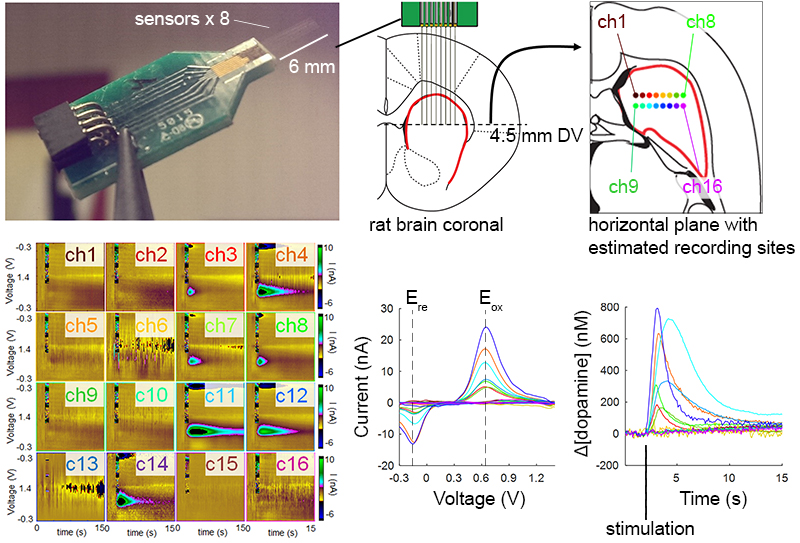
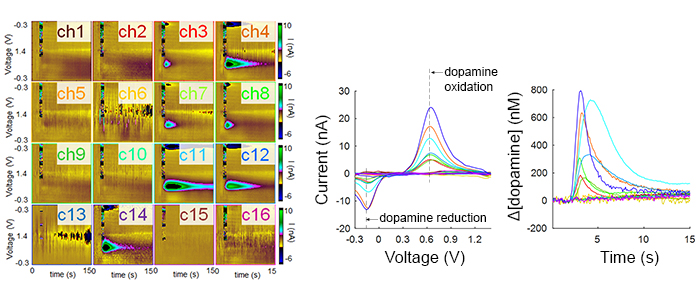
Most neurological disorders stem from chemical dysregulation at specific brain sites. Understanding the site-specific changes in dopamine signaling is crucial to elucidate the basis for many diseases, including Parkinson's disease, major depressive disorder and other mood disorders, and many other debilitating conditions.
We developed micro-arrays capable of measuring subsecond dopamine release from a wide distribution of sites in the brain and found highly heterogeneous dopamine dynamics in the rat striatum.
references:
Schwerdt et al., Subcellular probes for neurochemical recording from multiple brain sites, Lab Chip, 17(6), 2017.
Schwerdt et al., Subcellular electrode arrays for multisite recording of dopamine in vivo, in Proc IEEE MEMS, 2017.