|
Spotted Seatrout
Spawning Requirements and Essential Fish Habitat: A Microhabitat Approach
Using Hydrophones.
Donald M. Baltz. Oceanography and Coastal Sciences,
Coastal Fisheries Institute, Louisiana State University, Baton Rouge,
LA 70803, USA.
Hyrdrophones can be used in conjunction with a microhabitat approach
to yield a fish’s eye view of its habitat requirements. At the
finest scale, the microhabitat of an individual is the site it occupies
at a given point in time. Sites are presumably selected to optimize
an individual’s net energy gain while avoiding predators (i.e.,
tradeoffs of growth vs mortality). Since similarly sized individuals
of a species select similar microhabitats, many careful measurements
of individuals and associated physical, chemical, and biological variables
should define the population's responses to environmental gradients.
As defined here, the microhabitat is an occupied site, not a little
bitty habitat type. Fine-scale measurements of environmental conditions
at a site occupied by one or more individuals constitute an observation,
and many independent observations characterize the population’s
response to complex gradients.
Habitat is a loosely used ecological term that can be applied at the
individual, population, and community levels and is often entangled
with so many other ecological concepts that it can mean everything and
therefore nothing. The term ‘habitat’ has almost been relegated
to the status of a pseudocognate (sensu Salt 1979, Ecology) in that
it is a term in common use and each individual who uses it feels that
all others share his own intuitive definition. Nevertheless, it is used
and can be useful. We can use habitat as the range of environmental
conditions in which a species/population/life-history stage can live.
It is a general term that broadly defines where a species lives without
specifying patterns of resource use (sensu Hurlbert 1981, realized niche
= resources used: energy, materials, and sites (Evolutionary Theory
5:177-184)). There are several points of view. From a fish's point of
view, its distribution over environmental gradients describes its habitat.
From a biologist's point of view, strata in the environment can be arbitrarily
described as habitats, but more properly as "habitat types".
At the community level, the environments dominated by a single species
(e.g., Spartina alterniflora) may be characterized as Spartina habitat,
but more properly as a "Spartina community".
The concept of Essential Fish Habitat (EFH) is based on 1996 federal
legislation and is aimed at enhancing the sustainability of our fisheries.
The legislation established four levels of data quality in defining
EFH: I. Presence/absence, II. Density patterns (e.g., population responses
to gradients, suitability), III. Condition/health (e.g., growth, parasite
loads, pollution loads, RNA/DNA ratios), and IV. Production (e.g., secondary
production, reproductive output). What is EFH? Essential is a qualifier
that carries a notion of quality. We are not just asking where a species
lives (Level I), but where it lives well (Levels II-IV). Where are its
resource needs best met? Now we are concerned with patterns of resource
use (sensu Hurlbert 1981, realized niche = resources used: energy, materials,
and sites (Evolutionary Theory 5:177-184)).
How can we use a fish’s eye view to define EFH? We can seek answers
to three questions. How are species and life history stages distributed
in the environment? What intervals along environmental gradients are
selected or avoided? What is most important to the fish in terms of
growth and/or survival?
Level I data: Presence/Absence data. Most risk averse approach to
protecting habitat (based on the precautionary principle); however,
reliance on Level I data overprotects less valuable habitat and essentially
equates water with EFH. High quality habitat is given the same level
of protection as Low quality habitat and scientists/managers lose credibility.
Level II data: Density data. Uses fish population’s responses
(density patterns) to environmental gradients. Level II assessments
can be improved by relating population’s response in terms of resource
use to resource availability (e.g., habitat suitability), and high quality
can be distinguished from low quality habitat. Habitat Suitability [S
= Suitability = P (E | F) / P (E)] is an index of habitat quality based
on a quotient of Resource Use and Resource Availability (Bovee, K. D.
& T. Cochnauer, 1977, U.S. Fish & Wildlife Service Biological
Services Program FWS/OBS-77/63). Resource use is a probability statement,
given the presence of fish, and resource availability is a probability
statement, regardless of the presence of fish. Suitability is an index
of use divided by availability that ranges from zero (intolerable) to
one (optimal) after standardization.
Level III data: Growth data. I have not been able to relate this
level to hydrophone work on spotted seatrout, but others may find an
application for other soniferous species that make sounds for non-reproductive
functions (e.g., foraging parrotfishes). What environmental conditions
foster growth of early juvenile spotted seatrout? Nursery microhabitat
selection is presumably controlled by some combination of physiological
constraints, prey distributions, foraging success, competitor densities,
and predation pressure, all of which may influence growth and/or survival.
Linkages between microhabitat, diet, & conspecific density may predict
recent daily growth which in turn reveals the recruitment potential
of preferred nursery characteristics (Baltz et al.1998, Env Biol Fish
53: 89-103).
Level IV: Production data. This is the best kind of information
and the best example if from a study of oyster seed production (Chatry,
Dugas, and Easley 1983, Cont. Mar. Sci. 26: 81-94). Oyster seed set
and growth are best at 20-22 ppt (Level III data), but oyster predators
(drills, etc) seriously deplete populations in high salinity water (>
15 ppt). Oyster seed production is highest for seed set at 12-16 ppt
the previous summer, and therefore EFH for oyster seed production is
highest in a narrow summer salinity range of 12-16 ppt.
I will argue that suitability indices for reproduction (Saucier &
Baltz, 1993, Env Biol Fish 36: 257-272) are high-level EFH data and
qualify at Level IV. Moreover, this kind of data can be acquired easily
with hydrophones. Saucier and Baltz (1993) used a microhabitat approach
to identify selected and avoided points along salinity, depth, substrate,
and velocity gradients used for spawning by spotted seatrout. Relatively
deep, moving waters with a salinity of 14-23 and a temperature of 29-33
°C were selected. Conflicting literature from earlier studies in
the northern Gulf of Mexico suggested that spawning occurred more or
less exclusively in bays, passes or the open gulf. We found that spotted
seatrout spawn across a wide variety of habitat types (bays, channels,
passes, and open gulf) where environmental conditions are right. We
found that spawning locations shifted along a salinity gradient up to
30 km on a north-south axis, and concluded that environmental conditions
were more important than places.
|
Spawning temperatures for Louisiana spotted seatrout |
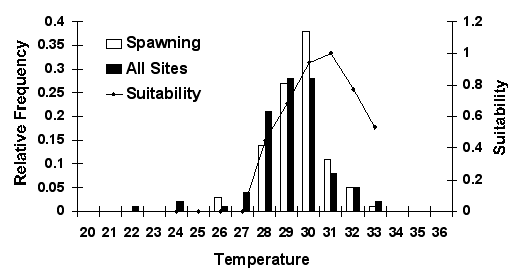 |
|
Spawning salinity for Louisiana spotted seatrout |
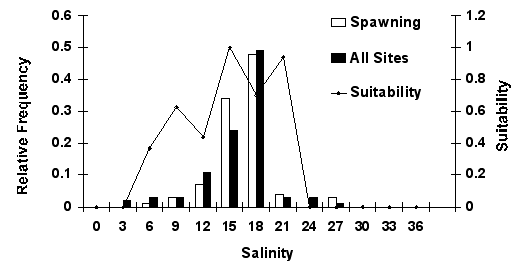 |
There are several
pitfalls to avoid. Non-linear effects along environmental gradients
should be expected. Non-representativeness in sampling design may lead
to biased results, especially sampling bias that focuses on particular
habitat types may generate misinformation. Noisy crews, boats and traffic
may make it difficult to locate spawning aggregations. Misidentification
of drumming species can be avoided by careful comparisons with known
recordings, and verification of actual spawning by the collection and
rearing of eggs from drummng sites to identifiable larvae is important.
A stratified water column may present contrasting environmental variables
in a vertical profile (We want to know what’s going on at fish’s
nose).
Hydrophone Techniques & Assumptions. Aggregation size: Sound
Intensity may be used to estimate Source Level (SL) if distance to source
is known. Source Level is calculated by adding a one-way spherical spreading
loss (i.e., a 20 log [depth in meters - 1]) for a correction (absorption
is ignored). It is a continuous variable that estimates group size for
statistical modeling: SL = microhabitat variables + temporal variables
+ e. A recorded Sound Intensity (my standard settings were 132 db re
1 m pascal) of +5 db yields a Source Level of 139.6 db for an aggregation
on the bottom in 15 m of water [e.g., 132 + 5 + (20 log 14) = 139.6
db]. A cylindrical correction or no correction may be more appropriate
under given circumstances.
Future Applications. My wish list is topped by a fixed or moveable
listening array with overlapping directional capabilities to generate
position fixes, computer programs to process fix data, and real-time
transmission capabilities to allow a small boat to move to aggregation
sites and random sites for measurements of resource use and availability.
Recommendations for EFH. Quality research and wise management
related to fish habitat depend on how clearly we can define habitat
and EFH and that we are all discussing the same concepts. We should
try to take a fish’s point of view and let them describe what is
essential along environmental gradients. By comparing resource use with
environmental availability, we gain insights into patterns of selection
and avoidance. We can identify biological endpoints that reflect the
health and well being of individuals and communities of fishes. Use
of the best data and research designs available will help avoid management
errors in describing EFH. Management errors that result in over- or
under-protecting EFH can be viewed as having positive and negative outcomes:
Over-protection
Species & habitats are better protected (+)
Scientists & managers may lose credibility (-)
Costs the regulated group its profits (-)
Enforcement is more expensive (-)
|
Under-protection
Species & habitats at greater risk (-)
Scientists & managers may lose credibility (-)
Regulated group is happy (+)
Enforcement is less costly (+) |
Clearly, we can use hydrophones and a microhabitat approach to credibly
describe EFH for some soniferous spawning fishes, like spotted seatrout.
Acknowledgments. I am grateful to Grant Gilmore and Mike Mok for
an acoustical primer and other assistance, Scott Holt for showing us
how to rear larvae, Louisiana Sea Grant for funding, and Rodney Rountree,
Tony Hawkins, & Cliff Goudey for putting together the workshop.
Return
to Top | Workshop Proceedings: Short
Papers
|

