Workshop Proceedings: Short Papers
Characterization of sounds and their use in two sciaenid species: weakfish and Atlantic croaker.
Martin A. Connaughton1, Michael L. Lunn1, Michael L. Fine2, & Malcolm H. Tayor3
1Washington College, 300 Washington Ave., Chestertown, MD 21620 mconnaughton2@washcoll.edu, milunn@yahoo.com
2Department of Biology, Virginia Commonwealth University, Richmond, VA 23284-2012, USA, mfine@atlas.vcu.edu.
3College of Marine Studies and Department of Biological Sciences, University of Delaware, Newark, DE 19716, USA, mtaylor@udel.edu.
Introduction
Both weakfish Cynoscion regalis and Atlantic croaker Micropogonias undulatus are members of the family Sciaenidae, a group of fish that have been known to produce sound since the turn of the 20th century. This family of fishes produces sound through the use of highly specialized, extrinsic sonic muscles, which lie in close proximity, but are not attached to the swimbladder . In most sciaenids, such as weakfish, sonic muscles are typically found only in the male; however, in others, including Atlantic croaker, the muscles are found in both males and females . Sound production has been linked to reproductive behavior in a number of sciaenid species and with fright or warning behaviors in a few species, including Atlantic croaker . The purpose of this paper is to characterize the sounds produced by two species of sciaenid and to discuss the roles of these sounds in the behaviors of these species.
Weakfish experiments
Field recordings Field recordings using a hydrophone (Edmund Scientific) were made near the mouth of the Delaware Bay at three stations along an inshore-offshore transect ranging from 1.24 to 5.64km from shore and varying in depth from 3.5 to 7.8m. One-minute recordings were made at hourly intervals over a 24hr period on eight dates from mid-April through mid-August, encompassing the late spring-early summer spawning season. Recordings of drumming sounds were ranked qualitatively from 0 - 4, with 0 representing no calls and 4 representing continuous calling by a chorus of individuals .
Drumming was found to be highly seasonal, increasing dramatically from zero in mid-April to nearly maximal levels in early May. Activity remained at near maximal levels throughout May and June, decreasing gradually through July and into August. Physiological indicators of reproductive readiness, including plasma androgen levels, male GSI, and the presence of hydrated eggs were all high during the period of maximal drumming activity. Drumming activity also expressed diel trends, reaching maximal levels between 20:00 and 24:00hr (sunset was between 19:50 - 20:40) and declining to a minimum between 05:30 and 10:30hr. Drumming activity, whether seasonal or diel, was most intense inshore, declining in intensity as one moved offshore . The seasonality, evening timing, and inshore location of sound production all coincide with the known reproductive activity of weakfish in this area .
Captive spawning recordings Captive weakfish held in a 1500L tank were induced to spawn with two injections of 1000 IU hCG/kg body weight administered in the early afternoon on two successive days. Fish spawned during the evening of the second day of injections. Spawning activity was documented on standard VHS tape with video (Ikegami CCD camera, ICD 4224) and audio (Edmund Scientific) input . Field and captive sounds, staccato bursts of 6-10 individual pulses, were identical . It was also determined that dominant frequency and repetition rate vary with temperature and fish size .
During courtship, only pair spawning was observed, though larger groups in larger enclosures might behave differently. Drumming activity was most often initiated after the first spawning event, but based on the timing of sound production and spawning in the field this observation may be due to a tank effect. The number of drumming bursts per minute varied somewhat between males, but remained relatively constant for a given male for the duration of the evening's sonic activity, i.e. number of bursts per minute did not drop off as time passed after a spawning event. Sound production ceased prior to gamete release, which was apparently synchronized by body contact.
Croaker experiments
Captive spawning recordings As above, field caught Atlantic croaker were maintained in laboratory tanks and induced to spawn following hormone injections, and video/audio recordings (B&W CCD camera, OS-40D, World Precision Instruments; model C21 hydrophone, Cetacean Research Technology) were made. To date, only a single successful trial, involving one male and two females, has been conducted. The courtship behavior of the croaker was similar to that exhibited by the weakfish: drumming began after the first spawning event, was maintained for several hours thereafter, and ceased just prior to gamete release.
Only the male produced courtship sounds, bursts of 1-3 pulses, with a mode of two pulses. Dominant frequency for the single recorded male (33cm total length) was 300Hz and the repetition rate of pulses within a burst was 5.4Hz. Courtship sounds were lower in frequency and repetition rate than fright response sounds (see below). In addition, the number of drumming bursts per minute decreased steadily following each spawning event, a behavioral characteristic not shared with weakfish (Fig. 1).
Fright response recordings Fright response recordings were made in a rectangular 1250L tank. Sound production was elicited by casting a shadow over the surface of the holding tank, or moving a dip net through the water. Thirteen fish, ranging in total length from 22.5 to 30cm were recorded, and both male and female croaker called readily.
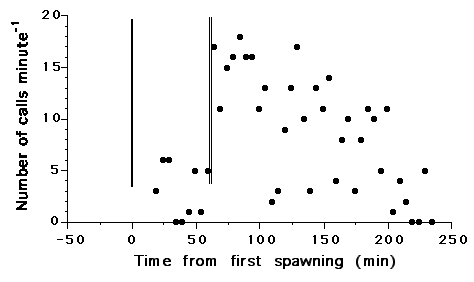 |
| Figure 1. Croaker courtship sound production expressed as number of calls min-1 across time. Values were determined for one minute out of every five recorded over the course of the evening. The solid vertical line represents the first spawning event (9:29PM) and the double vertical line, the second (10:29PM). |
Fright response calls varied more widely than the courtship calls, as the number of pulses per burst for each fright call ranged from 1-9, though the mode was still two. In contrast, the repetition rate of pulses within a burst was greater in fright response calls, ranging from 7.87 to 33.56 pulses/sec and expressing a mean of 18.09. Repetition rate was more variable in shorter bursts (2 or 3 pulses per burst) than in longer bursts (Fig. 2). Even given that dominant frequency appears to vary with fish size (650 to 540Hz for 22.5 to 29cm total length fish), courtship sounds appear to have a lower dominant frequency (approximately 100Hz lower for a 33cm fish) (Fig. 3).
Conclusions
Sound production in weakfish and croaker may be involved in the formation of spawning aggregations and/ or attracting a mate, though because of the small tank size, this could not be determined in our laboratory experiments . It may also play a role in female mate selection, since larger individuals of each species produce a sound with a lower dominant frequency .
Though weakfish will produce sounds if drawn to the surface when caught hook and line, or when removed from a tank into the air, we have never recorded a fright response call from weakfish like those so easily elicited from Atlantic croaker. In-air 'disturbance' calls elicited from weakfish when they are removed from the water were identical to courtship calls except for having a wider range of pulses in each burst of sound . In contrast, our data suggest that fright response and courtship calls in croaker may be quite distinct in dominant frequency and repetition rate, though more data needs to be collected.
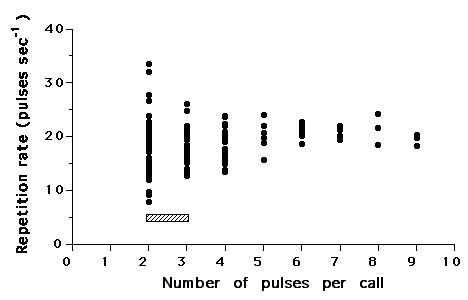 |
| Figure 2. Repetition rate plotted across number of pulses per call from sounds produced by male and female croaker (N=13) during fright response behaviors. The hatched block represents the repetition rate and number of pulses observed during courtship sound production. |
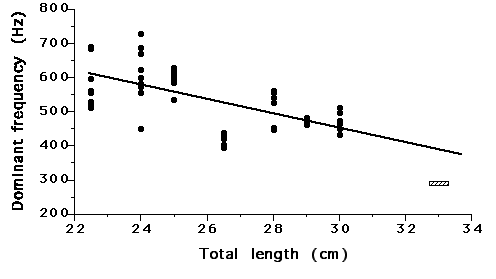 |
| Figure 3. Dominant frequency of individual sound pulses plotted across specimen total length from sounds produced by male and female croaker (N=13) during fright response behaviors. The hatched block represents the dominant frequency of calls made by the single male (33 cm) during courtship sound production. |
Acknowledgements
This work was supported by the Wallop-Breaux Sport Fishing Act with funds administered through the Delaware Department of Natural Resources and Environmental Control and by in-house funding provided by Washington College. The sponsors funded participation by M. A. Connaughton in the workshop.
References
Connaughton, M. A. and Taylor, M. H. (1995). Seasonal and daily cycles in sound production associated with spawning in the weakfish, Cynoscion regalis. Env. Biol. Fish. 42, 233-240.
Connaughton, M. A. and Taylor, M. H. (1996). Drumming, courtship, and spawning behavior in captive weakfish, Cynoscion regalis. Copeia 1996, 195-199.
Connaughton, M. A., Taylor, M. H. and Fine, M. L. (2000). Effects of fish size and temperature on weakfish disturbance calls: implications for the mechanism of sound generation. J. Exp. Biol. 203, 1503-1512.
Fish, J. F. and Cummings, W. C. (1972). A 50-dB increase in sustained ambient noise from fish (Cynoscion xanthulus). J. Acoust. Soc. Am. 52, 1266-1270.
Fish, M. P. and Mowbray, W. H. (1970). Sounds of western North Atlantic fishes. Baltimore: The Johns Hopkins Press.
Guest, W. C. and Lasswell, J. L. (1978). A note on courtship behavior and sound production of red drum. Copeia 1978, 337-338.
Mok, H. K. and Gilmore, R. G. (1983). Analysis of sound production in estuarine aggregations of Pogonias cromis, Bairdiella chrysoura, and Cynoscion nebulosus (Sciaenidae). Bulletin of the Institute of Zoology, Academia Sinica 22, 157-186.
Saucier, M. H. and Baltz, D. M. (1993). Spawning site selection by spotted seatrout, Cynoscion nebulosus, and black drum, Pogonias cromis, in Louisiana. Env. Biol. Fish. 36, 257-272.
Tavolga, W. N. (1964). Sonic characteristics and mechanisms in marine fishes. In Marine bio-acoustics, vol. 1 (ed. W. N. Tavolga), pp. 195-211. New York: Pergamon Press.
Taylor, M. H. and Villoso, E. P. (1994). Daily ovarian and spawning cycles in weakfish. Trans. Am. Fish. Soc. 123, 9-14.
Tower, R. W. (1908). The production of sound in the drumfishes, the sea-robin and the toadfish. Ann. N.Y. Acad. Sci. 18, 149-180.
Villoso, E. P. (1989). Reproductive biology and environmental control of spawning cycle of weakfish, Cynoscion regalis (Bloch and Schneider) in Delaware Bay: University of Delaware, Newark, Delaware.