Workshop Proceedings: Short Papers
Passive Acoustic Transects: Mating Calls and Spawning Ecology in East Florida Sciaenids
R. Grant Gilmore, Jr., Ph.D.
Dynamac Corporation, Mail Code: DYN-1, Kennedy Space Center, FL 32899,
USA.
rggilmorej@aol.com
Introduction
Historical Acoustic Work with Sciaenid Fishes: Sciaenid fishes have been known to produce sound for centuries (Aristotle, 1910; Dufossé, 1874a,b) and the association of sciaenid sounds with spawning has been known nearly as long (Darwin, 1874; Goode, 1887). For hundreds of years the Chinese have isolated sciaenid spawning sites from their water craft by listening to drumming sounds emanating from the water through the hull of their boats (Han Ling Wu, Shanghai Fisheries Institute, pers. comm.). The isolation of sciaenid spawning sites using underwater technology is recent and dependent on the availability of underwater transducers, hydrophones, and acoustic recorders used to access and study underwater sounds (Fish and Mowbray 1970). Hydrophone tape recordings of vocalizations produced by large sciaenid aggregations during spawning was pioneered by Dobrin (1947), Dijkgraaf (1947, 1949), Knudsen et al. (1948), Protasov and Aronov (1960), Schneider and Hasler (1960), Tavolga (1960, 1981), Fish and Mowbray (1970), Fish and Cummings (1972). The first isolation and description of soniferous sciaenid aggregations using mobile hydrophones moving along a sound transect at spawning sites was conducted by Takemura et al. (1978), Mok and Gilmore (1983) and Qi et al. (1984). A portable hydrophone and recording system was carried via a boat from one site to another along a measured transect with recordings made along a preset grid or in a linear series (Mok and Gilmore 1983; Gilmore 1994, 1996, 2002). Recordings were made for 30 - 300 seconds at each site depending on transect length. Recorded sounds were verified by recording captured specimens identified to species and documenting specific sound types through sonographic analyses. This technique allowed spatial-temporal isolation and identification of species-specific sounds produced by sciaenid fishes, particularly under conditions of high sound attenuation for large group sounds (low frequency high intensity sounds). Using detailed sonographic analyses of field recordings made on transects Mok and Gilmore (1983) described the characteristic sounds of black drum, Pogonias cromis, spotted sea trout, Cynoscion nebulosus and silver perch, Bairdiella chrysoura. Subsequent to these observations considerable additional work has been done on sound characterization in these species as well as the weakfish, C. regalis and the red drum, Sciaenops ocellata. Passive acoustic transect techniques have been used by several investigators to isolate spawning sciaenid groups in the field (Sausier and Baltz, 1992, 1993; Connaughton and Taylor, 1994, 1995; Luczkovich et al., 1999, 2000).
Recent East Florida Research 1990-2002: Over the past twelve years the value of passive acoustic studies in determining spatial and temporal spawning activity in sciaenids has increased. East central Florida studies have been supported by the Florida Fish and Wildlife Conservation Commission, U.S. Geological Survey, National Aeronautics and Space Administration, Canaveral National Seashore and NOAA/National Marine Fisheries Service. The major objectives of these studies have been to develop new techniques and technologies to allow real time continuous monitoring of soniferous aquatic organisms. This included a prototype neural network to identify species specific sounds (Lin 1996), and remotely deployed underwater computer systems (HBOI ALMS; NASA PAMS) with hydrophones and physical sensors for environmental parameters to allow association of physical oceanography with acoustic activity.
Future Acoustic Research at the Kennedy Space Center: The long term objectives of this work at the Kennedy Space Center is to develop an acoustic and sensor array that will allow continuous monitoring of biotic acoustic activity in association with intra and interspecific interactions as well as climatic and oceanographic phenomena. An experimental acoustic arena is being developed in the marine protected areas within the secure zone of the NASA and U.S. Air Force launch complex at Cape Canaveral.
Function of Sound Production in Sciaenids
The most predictable and robust sounds produced by many fishes are those associated with reproduction. As in many soniferous animals, it is the male that must attract a mate and induce her to donate eggs for fertilization, and, therefore, it is often only the male that produces sound. Large choral aggregations of male sciaenid species are formed by spotted seatrout, weakfish, red drum and silver perch specifically to attract females with which to spawn. Since these male choral aggregations contribute no significant resources required by females except the males themselves (no male paternal care, no food, or nest sites) they are appropriately called seatrout "leks", such as those formed by aggregative birds and amphibians strictly for the purpose of reproduction (Höglund and Alatalo 1995). A lek is an arena to which females come and on which most of the mating occurs. An arena is a site on which several males aggregate but does not form the habitat normally used by the species for other activities such as feeding. Sciaenid leks are seasonal and are associated with a wide variety of environmental parameters that are favorable for egg, larval and adult survival. The acoustic properties of lek sites are undoubtedly favorable for mating call transmission and must have specific acoustic properties. Although many sciaenid spawning sites have been isolated to date, their acoustic properties have not been studied in detail (Mok and Gilmore, 1983; Saucier and Baltz, 1992, 1993; Luczkovich et al., 1999; Gilmore 2002). Aggregative calling only occurs at the appropriate time for spawning, facilitating successful mating, egg fertilization, egg/larval dispersal and survival.
Sciaenid Sound Production Mechanisms
The most robust and energetic sciaenid sounds are produced by sonic muscles indirectly or directly vibrating the membrane of the gas bladder. When a freshly captured, recently calling, male seatrout is dissected, the bright red sonic muscles surrounding the gas bladder can be easily differentiated from the exterior lateral body muscles. The muscle vibratory rate is directly associated with the fundamental frequency of the characteristic seatrout call produced by the gas bladder.
Most of the 1,200 species in this family produce sound using sonic muscles associated with the gas bladder. Using the species specific muscle contraction rates and the gas bladder shape sciaenids produce diagnostic sounds that can be used to identify species within the family (Mok and Gilmore 1983), as has been demonstrated in amphibians and birds. The characteristic shape of the sciaenid gas bladder is so conservative that it has been used as one of the primary characters to classify sciaenids and to determine their phyletic relationships (Chu 1963; Chao 1978, 1986).
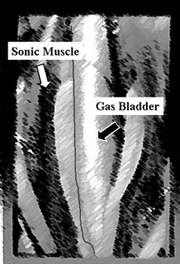
Representative sciaenid internal anatomy revealing the gas bladder and sonic muscles of a male weakfish, Cynoscion regalis.
Classification of Sciaenid Sounds.
The following figures illustrate the diagnostic mating calls of sciaenids known to spawn in the Indian River Lagoon system of east central Florida.
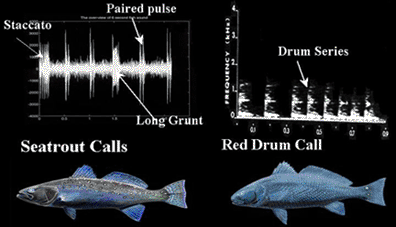
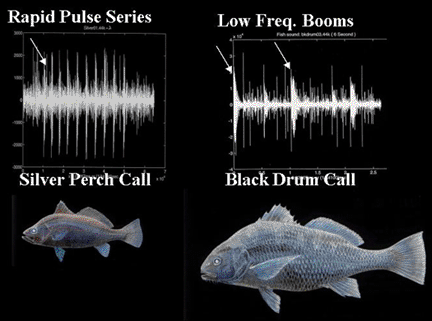
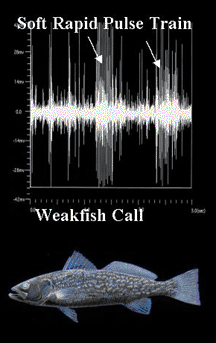
Energy distribution patterns associated with harmonic frequency bands and fundamental frequencies are species specific and were used to train a neural network to recognize sciaenid calls (Lin 1996).
Sciaenid Acoustic and Spawning Ecology: When and Where do Sciaenids Produce Sound?
Mok and Gilmore (1983) demonstrated that sciaenid sound production was specifically associated with crepuscular and nocturnal courtship and spawning activities. Pelagic eggs and larvae of the spotted seatrout were collected with plankton nets at spawning sites during vocalization periods (Mok and Gilmore, 1983; Alshuth and Gilmore, 1993, 1994, 1995). These same studies of soniferous spawning aggregations have demonstrated long-term spawning site fidelity, with the principal spawning sites identified by Mok and Gilmore (1983) being used for over twenty years (Gilmore, 1994, 1996, 2002).
As male spotted seatrout could be recognized by distinctive crepuscular calls their presence or absence from specific locations could be determined and the percent occurrence of calls at all acoustic listening sites derived. In addition, the approximate size of the calling group could be estimated based upon sound intensity (dB level, re 1 mPa) and group size estimates using a three part scale: 1 - small group or individual callers; 2 - moderate groups of several tens of callers; and 3 - a large group of what appear to be hundreds of simultaneous callers. Unfortunately, mixed species chorus behaviors were common with Arius felis and Bairdiella chrysoura joining in with spotted seatrout calls, therefore, elevating site specific sound intensities and masking seatrout numbers based on sound intensity. The percent occurrence of spotted seatrout calls at a site or time period is the most objective data used to define site and period use by seatrout leks. However, Gilmore (1994) found that spatial and temporal distributions of the estimated group size, egg and larval abundance in the water column and percent occurrence of calling trout were highly correlated (r = 0.92 to 0.98 at a = 0.05). This indicates that group size estimates were a useful, independently derived variable that could be used to verify calling trout distributions and relative use of specific sites or particular times of the year. These two data types have been used to isolate spawning times and locations.
Seasonal mating calls were directly associated with primary spawning activity in east central Florida sciaenids. The following figure summarizes their seasonal call pattern at this latitude based on over 300 acoustic transects between 1978 and 2002.
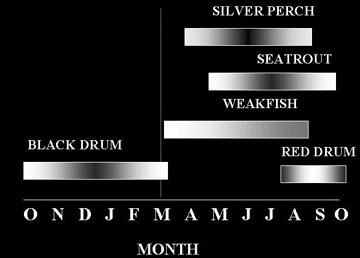
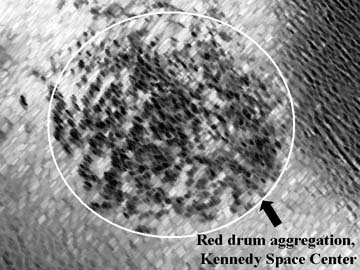
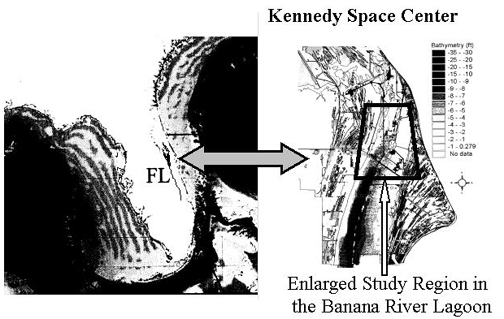
Spatial distribution of sciaenid spawning calls - All soniferous spawning populations of sciaenids in the upper Banana River Lagoon, a lagoon associated with the Indian River Lagoon system, within the protected waters of the Kennedy Space Center have been mapped. Spawning sites are utilized only from sunset to midnight during the spawning period with greater call activity on new and full moon phases. Some sites within the Indian River Lagoon system have been known as favored spawning sites with mating calls having been recorded from these sites for over 20 years. The following figure represents primary call sites for all sciaenids known to spawn in the upper Banana River Lagoon basin north of the NASA Causeway at the Kennedy Space Center.
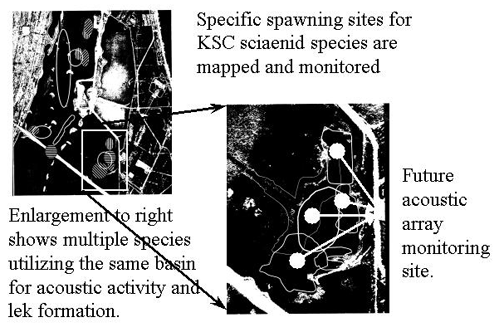
FUTURE TECHNOLOGY DEVELOPMENTS AT KSC RELATIVE TO ACOUSTIC STUDIES OF SCIAENID FISHES
Once locations and periods of acoustic activity and spawning have been isolated as they have at the Kennedy Space Center, then a number of basic questions and hypotheses can be addressed relative to the evolutionary significance of sound production in sciaenids and other soniferous fishes. Examples are:
1. Does sciaenid sound production increase the probability of predation mortality?
2. What are the energetic costs of sonifery?
3. How does sciaenid foraging behavior relate to spawning and sound production and are there sexual differences in foraging behavior as a result of differences in mating behavior, acoustic energetics?
4. What are the benefits of sonifery to spawning aggregations?
With the installation of permanent acoustic arrays, portable acoustic systems, roving robotic acoustic platforms and physical sensor arrays we believe it will be possible to address detailed research objectives that will finally unravel the intimate mating, foraging and predatory escape behaviors of regional estuarine sciaenid communities.
 |
| Sensor Web pod deployments (developed by Kevin Delin, NASA/JPL, Pasadena, CA) and our NASA/KSC PAMS (Portable Acoustic Monitoring System) being deployed by the Clelia submarine during a NOAA OE project August 2001. |
Literature Cited
Alshuth, S. and R.G. Gilmore. 1993. Egg identification, early larval development and ecology of the spotted seatrout, Cynoscion nebulosus C. (Pisces: Sciaenidae). ICES C.M. 1993/G 28, Dem. Fish Com: 18 pp.
___. 1994. Salinity and temperature tolerance limits for larval spotted seatrout, Cynoscion nebulosus C. (Pisces: Sciaenidae). ICES C.M. 1994/L:17, Biol. Oceanogr. Cttee., 19 pp.
____. 1995. Egg and early larval characteristics of Pogonias cromis, Bairdiella chrysoura, and Cynoscion nebulosus (Pisces: Sciaenidae), from the Indian River Lagoon, Florida. ICES C.M. 1995/L:17, Biol. Oceanogr. Cttee., 21 pp.
Aristotle. Historia Animalium, IV, 9. Trans. by D.D'A.Thompson. 1910. Oxford. Clarendon Press.
Chu, Y.T., Y.L. Lo and H.L. Wu. 1963. A study on the classification of the sciaenoid fishes of China, with description of new genera and species. 1972 Reprint, Antiquariaat Junk, Netherlands.
Chao, L.N. 1978. A basis for classifying western Atlantic sciaenidae (Teleostei: Perciformes). NOAA Tech. Rept. NMFS Circ. 415.
Chao, L.N. 1986. A synopsis on zoogeography of the sciaenidae. Pp. 570-589, In T. Uyeno, R. Arai, T. Taniuchi and K. Matsuura (eds.) Indo-Pacific Fish Biol.: Proceedings of the Second Internatl. Conf. Indo-Pac. Fishes.
Connaughton, M.A. and M.H. Taylor. 1994. Seasonal cycles in the sonic muscles of the weakfish, Cynoscion regalis. Fish. Bull. 92: 697-703.
Connaughton, M.A. and M.H. Taylor. 1995. Seasonal and daily cycles in sound production associated with spawning in the weakfish, Cynoscion regalis. Envir. Biol. Fish. 42: 233-240.
Darwin, C. 1874. The descent of man. 2nd edition. N.Y.: H.H. Caldwell.
Dijkgraaf 1947. Ein tone erzeugender fisch in Neapler Aquarium. Experimenta, vol. 3, pp. 494-494.
Dijkgraaf 1949. Untersuchungen uber die funktionen des ohrlabyrinths bei meeresfischen. Physiol. Comp. Oecolog., vol. 2, pp. 81-106.
Dobrin 1948. Measurements of underwater noise produced by marine life. Sci., Vol. 105: 19-23.
Dufossé, A. 1874a. Rescherches sur les bruts et les sons expressifs que font entendre les poissons d'Europe et sur les organes producteurs de ces phenomenes acoustiques ainsi que sur les appareils de l'audition de plusieurs de ces animaux. Annales des Sci. Naturelles ser 5, 19: 53 pp.
Dufossé, A. 1874b. Rescherches sur les bruts et les sons expressifs que font entendre les poissons d'Europe et sur les organes producteurs de ces phenomenes acoustiques ainsi que sur les appareils de l'audition de plusieurs de ces animaux. Annales des Sci. Naturelles ser 5, 20: 134 pp.
Fish, J.F. and W.C. Cummings. 1972. A 50-dB increase in sustained ambient noise from fish (Cynoscion xanthurus). J. Acoust. Soc. Amer. 52: 1266-1270.
Fish, M.P. and W.H. Mowbray. 1970. Sounds of western North Atlantic fishes. The Johns Hopkins Press, Baltimore.
Gilmore, R.G.,, Jr. 1994. Environmental parameters associated with spawning, larval dispersal and early life history of the spotted seatrout, Cynoscion nebulosus (Cuvier). Final Program Rev., Contract No. LCD 347. Mar. Res. Inst., Fla. Dept. Environ. Protection, St. Petersburg, Fla.
Gilmore, R.G. , Jr. 1996. Isolation of spawning groups of spotted seatrout, Cynoscion nebulosus, using passive hydroacoustic methodologies. Final Report, 21 November 1996, 12 pp., Fla. Dept. Environ. Protection, Mar. Res. Inst., St. Petersburg, Fla. Subcontract to Dr. Roy Crabtree, Principal Investigator, FMRI study entitled: The reproductive biology of spotted seatrout, Cynoscion nebulosus, in the Indian River Lagoon.
Gilmore, R.G, Jr.. 2000. Spawning site fidelity, classification of spawning environments and evolutionary stable strategies: Serranids versus Sciaenids. (Abstract) In Session 54: Fish Aggregation Symposium. 80th Annual Meeting Am. Soc. Ichthy. And Herp., La Paz, Mexico, June 14-20, 2000.
Gilmore, R.G, Jr.. 2002. Sound production and communication in the spotted seatrout. Chapter 11. pp. 177-195, in Biology of the Spotted Seatrout, S.A. Bortone, ed. CRC Press, Boca Raton.
Goode, G. B. 1887. American fishes: a popular treatise upon the game and food fishes of North America with special reference to habits and methods of capture. L.C. Page Co., Boston.
Hoglund, J. and R.V. Alatalo. 1995. Leks. Princeton Univ. Press. 248 pp.
Knudsen, V.O. , R.S. Alfred and J.W. Emling. 1948. Underwater ambient noise. J. Mar. Res., Vol. 7: 410-429.
Lin, Ya Di. 1996. A neural network for the isolation of fish sounds. Ph.D. Dissertation, Florida Institute of Technology, Melbourne, Florida, USA.
Luczkovich, J. J., M.W. Sprague, S.E. Johnson and R.C. Pullinger. 1999. Delimiting spawning areas of weakfish, Cynoscion regalis (family Sciaenidae), in Pamlico Sound, North Carolina usign passive hydroacoustic surveys. Bioacoustics 10: 143-160.
Luczkovich, J.J., H.J. Daniel III, M. Hutchinson, T. Jenkins, S.E. Johnson, R.C. Pullinger and M.W. Sprague. 2000. Sounds of sex and death in the sea: bottlenose dolphin whistles suppress mating choruses of silver perch. Bioacoustics 10. 323-334.
Protasov, V.R. and M.I. Aronov 1960. On the biological significance of sounds of certain Black Sea fish. (In Russian). Biofizika, Vol. 5: 750-752.
Qi, M., S. Zhang and Z. Song. 1984. Studies on the aggregate sound production of most species of croaker (sciaenoid fishes) in Bohai Sea, Yellow Sea and East China Sea. Studia Marina Sinica, Peking, 21: 253-264.
Saucier, M.H. and D.M. Baltz. 1992. Hydrophone identification of spawning sites of spotted seatrout Cynoscion nebulosus (Osteichthys: Sciaenidae) near Charleston, South Carolina. N.E. Gulf Sci. 12(2): 141-145.
Saucier, M.H. and D.M. Baltz. 1993. Spawning site selection by spotted seatrout, Cynoscion nebulosus and black drum, Pogonias cromis, in Louisiana. Env. Biol. Fishes 36: 257-272.
Schneider, H. and A.D. Hasler 1960. Laute and luerzeugung beim susswassertrommler Aplodinotus grunniens Rafinesque (Scianenidae, Pisces), Zeitschr. Vergl. Physiol., Vol. 43: 499-517.
Takemura, A, T. Takita and K. Mizue. 1978. Studies on the underwater sound - VII: Underwater calls of the Japanese marine drum fishes(Sciaenidae). Bull. Jap. Soc. Sci. Fish. 44: 121-125.
Tavolga, W. N. 1960. Sound production and underwater communication in fishes. pp. 93- 136, in Animal Sounds and Communication, Lanyon, W.E. and W. N. Tavolga, eds., AIBS 7.
Tavolga, W.N., A.N. Popper and R.R. Fay. (eds). 1981. Hearing and sound communication in fishes. Springer Verlag, N.Y.: pp 395-425.