|
Passive Detection
and Localization of Transient Signals from Marine Mammals using Widely
Spaced Bottom Mounted Hydrophones in Open Ocean Environments
Susan Jarvis and David Moretti
Naval Undersea Warfare Center Division Newport
Engineering, Test and Evaluation Department
1176 Howell St., Newport, R.I. 02841-1708
Abstract: One objective of the Marine Mammal Monitoring on Navy
Ranges project is to use existing Navy undersea range infrastructure
to develop a toolset for passive detection and localization of marine
mammals. The Office of Naval Research funded the M3R project as part
of the Navy’s effort to determine the effects of acoustic emissions
on marine mammals and threatened/ endangered species. A necessary first
step in this effort is the creation of a baseline of behavior which
requires long-term monitoring of marine mammals. Such monitoring, in
turn, requires the ability to detect and localize the animals. This
paper will present algorithms for passive detection and localization
of transient signals developed as part of the M3R toolset. It will also
present results of the application of these tools to detection and tracking
of various toothed whales at the Atlantic Undersea Test and Evaluation
Center (AUTEC), Andros Island, Bahamas.
1.0 Introduction
Navy undersea ranges such as the Atlantic Undersea Test and Evaluation
Center (AUTEC) use arrays of widely spaced bottom mounted hydrophones
to acoustically track undersea and surface vehicles. Traditionally,
the vehicles are equipped with acoustic pingers that emit known identification
signals at known repetition rates. Increasingly, range instrumentation
infrastructure (figure 1) is being applied to non-traditional tracking
problems. The Marine Mammal Monitoring on Navy Undersea Ranges (M3R)
program has developed a set of signal processing tools to detect and
track marine mammals using Navy range facilities [1]. Under the M3R
program, algorithms were developed to automatically detect and track
two classes of whale vocalizations -- clicks and whistles. Both of these
classes of signals are transient in nature. The tool set was recently
tested at AUTEC over a two-week period. Over five hundred square nautical
miles of ocean were simultaneously monitored via 68 broad-band hydrophones.
Several species of toothed whales where automatically detected and tracked
in real-time. The positional accuracy of the M3R tracking tools was
confirmed by visual sightings by a surface craft and by manual
analysis of the hydrophone data.
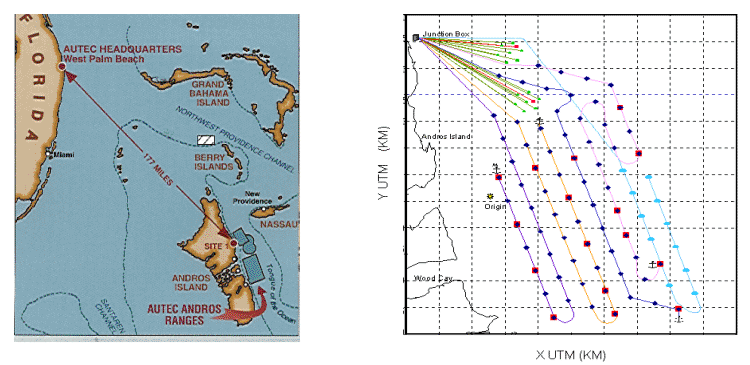 |
| Figure 1:
AUTEC has sixty eight, broad-band bottom mounted hydrophones with
a 2 NMi baseline. |
2.0 Discussion
Visual sightings [2] and aural analysis of hydrophone recordings [3]
indicate that many species of toothed whales are present at AUTEC. The
most commonly seen and heard are sperm whales, dolphins and short finned
pilot whales, all of which are present nearly year round. Consequently,
the M3R tools were developed to detect and track these common species.
For purposes of algorithm development, the vocalizations produced by
the whales were characterized as clicks and whistles. Clicks are, in
general, any impulsive, broad-band signal. However, sperm whale clicks
were of particular interest. Sperm whale clicks are very distinct, have
high source level, and occur in regular patterns [4] or "click
trains" (figure 2). Whistles were more broadly defined as any narrowband
event that sweeps in frequency over time (figure 3).
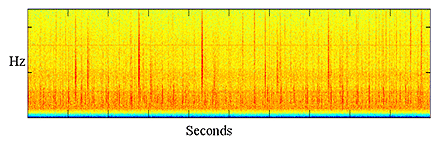 |
Figure
2: Spectrogram showing sperm whale clicks from several individuals |
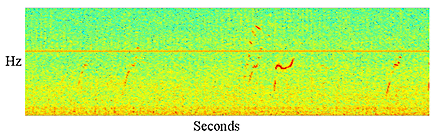 |
Figure
3: Spectrogram showing a sequence of whistles probably from a short
finned pilot whale |
There are three distinct parts to the whale localization problem. First
the vocalizations or whale calls must be automatically detected. In
order to determine the position of the animal in three dimensions, a
given call must be detected on a minimum of four hydrophones. The second
part of the problem is the association of the detections received on
various hydrophones with each other. That is, one must be able to determine
that the call received on hydrophone A at time tA is the
same signal that was received by hydrophone B at time tB.
Finally, associated sets of arrival times are input into a multilateration
algorithm to solve for position. A three-dimensional hyperbolic positioning
model [5] is used to determine the vocalizing animal’s location
in X, Y, and Z as well as the time of emission of the call.
M3R employs a real-time frequency domain energy detector for whale call
detection. A spectrogram of the incoming acoustic data from each of
the hydrophones is formed using 512-point fast Fourier transforms (FFT)
with a rectangular window and fifty percent overlap. The resultant spectrogram
has a frequency resolution of approximately 51 Hz and a time resolution
of approximately 9.8 ms. Each time-frequency bin of the spectrogram
is compared to a time varying threshold, D(f,t). The threshold
is set to be m dB above the (time) average power within frequency bin
f. The output of the detector, Qi(f,t), for each hydrophone,
is a binary valued "detection spectrogram" which contains
a 1 in each time-frequency bin that exceeded D(f,t) and a 0 everywhere
else (figure 4). The detection spectrogram indicates, in real-time,
the presence of whale vocalizations as well as providing information
on their frequency content. As evident in figures 2 and 3, the signal
structure of (sperm whale) clicks is very different from the signal
structure of whistles. Therefore, separate detection association algorithms
were developed for each signal type.
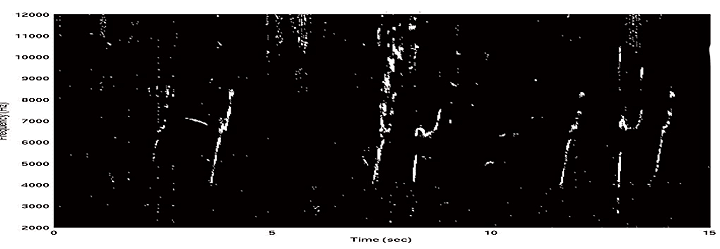 |
| Figure 4: Detection spectrogram, Q(f,t), of
whistles. Threshold was set 6-dB above the average spectral power. |
A series of clicks from a single sperm whale exhibits nearly the same
inter-click interval on all receiving hydrophones. Calls from each additional
animal exhibit their own unique pattern. In fact, inter-click interval
patterns were found to be an effective means of both differentiating
between individual whales and associating patterns of detections among
hydrophones [6]. In the first step of the M3R click association algorithm
the time-frequency detection spectra from all hydrophones were reduced
to binary "click maps". Click maps contain a 1 for time indices
where broad band events occurred in the detection spectrum and 0 for
all other times (figure 5). Conceptually, the next step is to cross
correlate the click maps from several hydrophones with a master hydrophone
to find the difference in time of arrival between each hydrophone and
the master. However, care must be taken in implementing the cross correlation
in order to properly associate each click detection among the hydrophones.
Figure 6a shows an example of a click map from a single hydrophone containing
clicks from two individuals. Figure 6b shows the click map from a second
hydrophone over the same time period. The question is which clicks in
figure 6b belong to which individual?
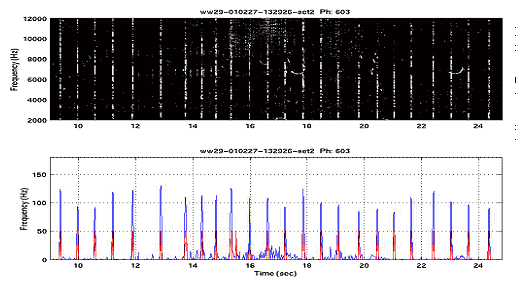 |
Figure
5: Click maps are formed by summing the detection spectrum (above)
along frequency then thresholding the sums. The red curve indicates
the click map for this data. |
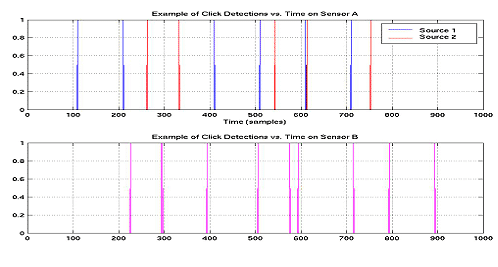 |
Figure
6: Click maps for two hydrophones containing clicks from two individuals.
It is not evident which clicks received by hydrophone B are associated
with Source 1 or Source 2 |
The M3R click association algorithm uses the notion of a "scanning
sieve" [7] to match detection patterns between hydrophones. The
sequence of click detections (i.e. click map) within the scanning sieve
on the master channel is compared to the clicks maps from surrounding
hydrophones ("scanned" signals). The scanning sieve time window
always starts on a click detection, and is moved across the scanned
signal one click detection at a time. That is, the resultant correlation
value at any time delay represents the number of matches between the
master channel pattern starting a specific click and the scanned channel
pattern starting at a specific click. The delay of the maximum correlation
value represents the difference in time of arrival where the first clicks
in the scanning sieve and the scanned signal were best aligned. The
output of the scanning sieve process are sets of time difference of
arrival (TDOA) for each click detection received on the master channel
(figure 7). The TDOA data from each hydrophone are then histogrammed
to estimate the number of separable sources (figure 8). Only detections
associated with significant populations are used (figure 9). These associated
TDOA sets are then sent to the AUTEC multilateration tracking algorithm
which calculates 3D position.
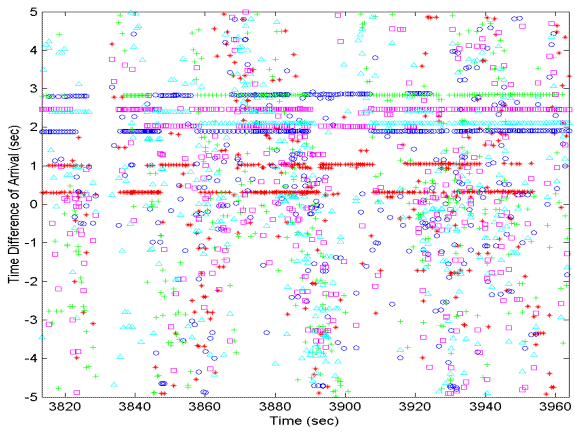 |
Figure 7:
Preliminary output of the M3R click association algorithm showing
the estimated TDOA between the scanning sieve, hydropohne 611,
and five additional hydrophones.
|
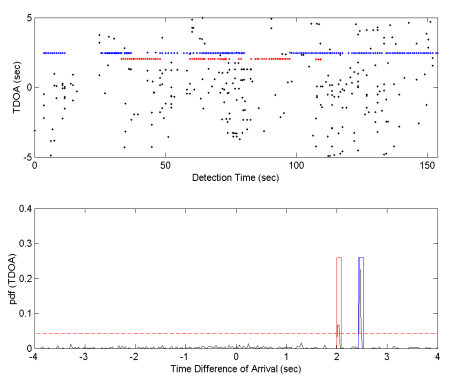 |
Figure
8: Above is the output of the click association algorithm for hydrophone
612 (indicated in purple in figure 7). Two separate times of arrival
are evident indicating the presence of 2 whales. A histogram of
the TDOA data shows two significant populations. |
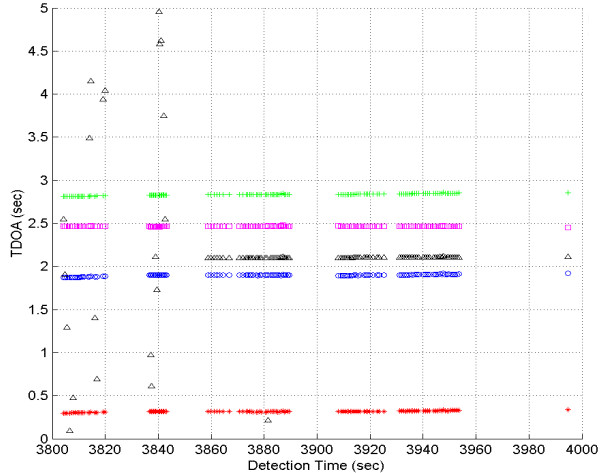 |
Figure 9: Final
output of the click association algorithm for calls from a single
sperm whale. The points indicate the TDOA at which the algrorithm
found the best matches between the scanning sieve and the clicks
maps of hydrophones 605, 603, 604, 612 and 606 relative to master
hydrophone, 611. Notice the minimal scatter of the TDOA points.
|
Whistle vocalizations
do not typically follow known repetition patterns. An individual can
emit a single short whistle or groups of sweeps that last several seconds
or both. However, the time-frequency characteristics of the calls in
whatever sequence they may occur remain the same on all receiving hydrophones.
To determine the TDOA of signals among the hydrophones, the detection
spectrograms Q(f,t) of the available hydrophones are cross-correlated
against a master channel, M [8]. The cross correlation Ci(t,
t) between the i-th channel and the master channel is calculated over
a time window of approximately 6 to 10 seconds. That time window is
then advanced by one half its duration and Ci(t,
t) is updated.
Ci(t, t)
= SSQM(f,t)Qi(f,t
+ t)
The time delay associated with the peak of the correlation functions
indicates the TDOA for a signal relative to the master hydrophone (figure
10a). If whistles from multiple whales are present within a cross correlation
time window, multiple correlation peaks will be evident (figure 10b).
Note that if both clicks and whistles are present at the same time,
sperm whale clicks will dominate the detection spectra. Correlation
peaks due to whistle signals will be obscured. Therefore, for practical
purposes, broad-band click events should be removed from the detection
spectra prior cross correlation.
While cross correlation of detection spectra indicates times of signal
arrival and the presence of multiple whales, it does not associate the
time delays of the correlation peaks with an individual across the hydrophone
channels. However, as mentioned early, the sequence of whistles from
an individual is the same on all receiving hydrophones. Figure 11 shows
the time differences of arrival relative to a master hydrophone of the
correlation peaks for five hydrophones. Notice that there are two distinct
patterns of detections versus time along specific time delays. Matching
these patterns along time delays associates the TDOA’s among the
hydrophones with an individual whale. Associated sets of TDOA can then
be the sent to the multilateration tracking algorithm which calculates
3D position.
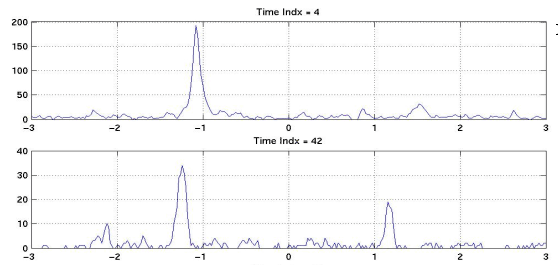 |
Figure 10:
a) Results of cross correlation indicating the TDOA of the signal
from one whale.
b) Cross
correlation results indicating the TDOA’s of signals from
two whales.
|
 |
Figure
11: TDOA data resulting from cross correlations of five hydrophones
against a master hydrophone. Two individual whales show two distinct
patterns (indicated by purple and green boxes) of detections versus
time along specific TDOA’s. |
3.0
Recent Results
The M3R toolset was demonstrated at AUTEC as part of a joint experiment
with researchers from Woods Hole Oceanographic Institution (WHOI). The
WHOI team was testing a new whale tagging system [9]. The M3R algorithms
were used with sixty-eight of the AUTEC hydrophones to monitor over
500 sq. NMi. When marine mammals were localized, their positions were
relayed to the tagging vessel, which then endeavored to maneuver close
enough to place a tag.
The detection, association and tracking algorithms described in Section
2.0 were implemented to run in real-time for arrays of five to seven
hydrophones. Given that there were 68 hydrophones to monitor, other
display tools were used to broadly locate whales before applying the
high resolution positioning algorithm. The Circle display is a Matlab
program that shows the number of detections on each hydrophone by drawing
a circle around the respective hydrophone. The number of detections
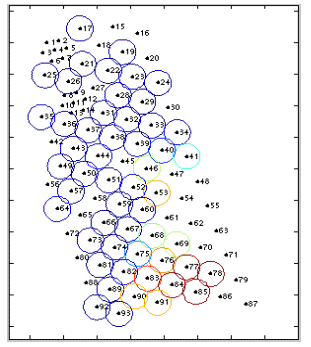
per minute is mapped to the color of the circle. Figure 12 shows an
example of the Circle display while two groups of whales were on the
range. The bright circles around Hydrophone 85 were caused by a single
clicking sperm whale. The bright circles around Hydrophone 53 were caused
by a group of pilot whales just off the range (hydrophones 47, 48, 54
and 55 were not monitored). The WHOI team successfully tagged two of
the pilot whales shortly after this picture was taken.
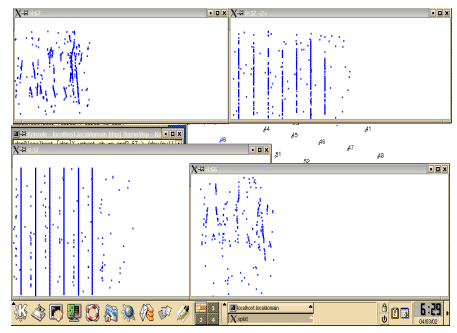
The strip chart program displays the detection spectrogram from a particular
hydrophone in real-time. The program reads the detection data from a
server process allowing the user to run multiple charts on multiple
computers simultaneously. During the AUTEC tests this display was quite
handy for monitoring phones over a wide area. At various times, both
broad sperm whales clicks, and pilot whale clicks and whistles were
evident (figure 13).
 |
|
 |
| Figure
12: Circle detection count display. maps color of the circle around
a hydrophone to the number of detection per minute. |
|
Figure
13: Strip chart displays scroll horizontally to display detection
spectra for a given hydrophone in real-time. The top right and lower
left charts show sperm whale clicks while the top left and lower
right show whistles. |
 |
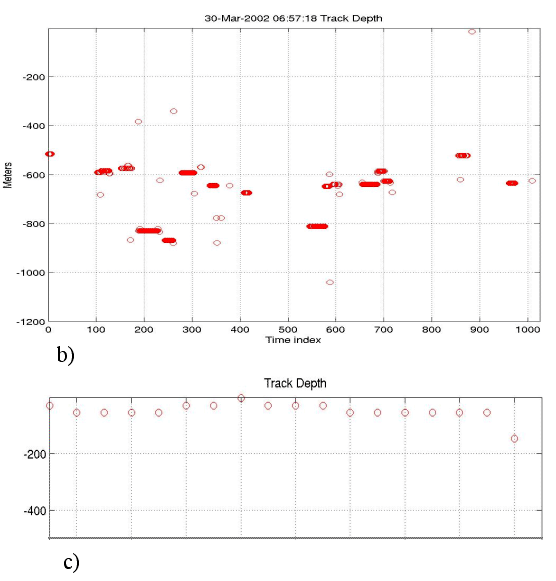 |
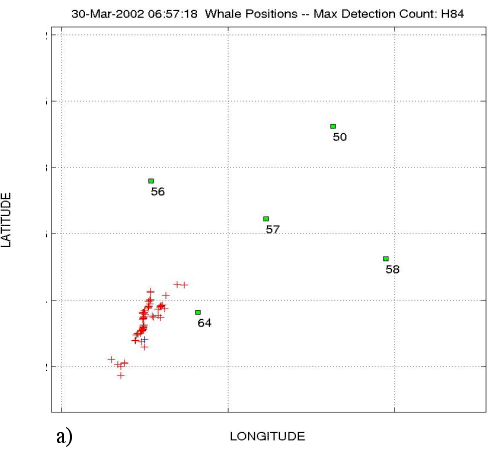
Figure
14: Examples of position and depth plots for sperm whales. a) Real-time
X-Y display for a group of 2 or 3 individuals. b) The depth plot
for that group.
c) A depth plot showing the shallow dives of a single sperm whale. |
|
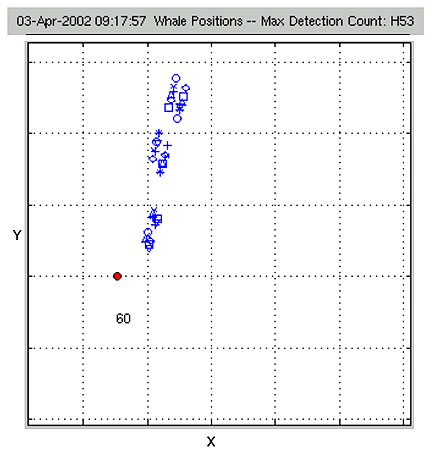 |
Figure
15: A real-time X-Y position plot for multiple whistlers. The icon
shapes indicate the individuals that the M3R tools were able to
identify. This group was observed by researchers aboard the WHOI
whale tagging vessel. The group consisted of more than twenty roughed
toothed dolphins and melon headed whales. |
4.0 Summary
The M3R project has developed algorithms for the passive detection and
tracking of marine mammals using widely spaced, bottom-mounted hydrophones
characteristic of Navy undersea tracking ranges. While these algorithms
have been implemented and tested for deployment at the AUTEC, they are
applicable to any fixed or portable range that uses multilateration
tracking algorithms. Potential ranges with the hardware to support the
M3R system include the Pacific Missile Range Facility, the Southern
California Offshore Acoustic Range, and any of the Navy’s various
portable systems.
The M3R algorithms have been designed to work in a highly channelized
multi-processor hardware environment, and the software architecture
has been developed to be fully network compatible. Signal detection,
and detection-association algorithms for two primary types of whale
calls, whistles and clicks, have been developed. These algorithms are
specifically designed to be used with widely spaced sensors, and assume
that the marine mammals vocalize repetitively with sufficient source
levels to be detected on multiple hydrophones.
The M3R algorithms, for both clicks and whistles, have been successfully
demonstrated resulting in real time 3D tracking of several species of
toothed whales including sperm whales, rough toothed dolphins, melon
headed whales and pilot whales. The M3R tool set allows automated collection
of data previously unavailable for the long-term monitoring of marine
mammal bioacoustics within their natural environment. This opportunity
has been created with minimal investment in infrastructure by providing
Navy ranges as a dual-use asset. Research applications of the M3R system
include the ability to remotely estimate marine mammal abundance, assessment
of bioacoustic behavioral baselines, and evaluation of the impact of
anthropogenic noise compared to those baselines.
Acknowledgement
We would like to acknowledge our sponsor, Dr. Robert Gisiner, at the
Office of Naval Research, for funding this project. We would also like
to thank the AUTEC personnel, especially Thomas Szlyk, for access to
their considerable infrastructure and ongoing support. In addition,
we would like to thank the NUWC Division Newport ILIR program manager,
Richard Philips, for supporting the initiative to create a baseline
sperm whale bioacoustic characterization in the Tongue of the Ocean
(TOTO).
References:
[1] Moretti, D., et al., "Marine Mammal Monitoring on Navy Ranges
(M3R)", Proceedings of the Undersea DefenseTechnology Hawaii 2001
Conference, October 2001.
[2] Naval Undersea Warfare Center Detachment AUTEC, "Final Environmental
Review, Adoption of a Range Management Plan for the Atlantic Undersea
Test and Evaluation Center (AUTEC), Andros Island, Bahamas", p.
57, September 1997.
[3] Perkins, P. J., "Bioacoustics at AUTEC, Bahamas: A Survey and
Guide", Naval Undersea Systems Center Technical Memorandum 87-2018,
1987.
[4] Jaquet N., Dawson S., and Douglas L., "Vocal behavior of male
sperm whales: Why do they click?", Journal of the Acoustical Society
of America, Vol.109(5), pp. 2254-2259, 2001.
[5] Vincent, H., "Models, Algorithms, and Measurements for Underwater
Acoustic Positioning", Ph.D. Dissertation. University of Rhode
Island, Kingston, R.I., 2001.
[6] Ward J., "Sperm Whale Bioacoustic Characterization at the Tongue
of the Ocean, Bahamas (U)," NUWCDIVNPT TM-01-106, Naval Undersea
Warfare Center, Division Newport, RI. December 2001 (Unclassified).
[7] Hu, J., and H. Vincent, "A Real-Time Multi-Hydrophone Data
Association Algorithm for Marine Mammal Transient Signals", NUWCDIVNPT
TM-01-107, Naval Undersea Warfare Center, Division Newport, RI. December
2001 (Unclassified).
[8] Moretti, D., et al., "Open Ocean Marine Mammal Monitoring Using
Widely Spaced Bottom Mounted Hydrophones (U)", to appear in Journal
of Underwater Acoustics, 2002.
[9] Tyack, P., et al., "Acoustic Response and Detection of Marine
Mammals Using and Advanced Digital Acoustic Recording Tag – CS1188",
www.serdp.org/reportong/reporting.html.
Return
to Top | Workshop Proceedings: Short
Papers
|
















