Workshop Proceedings: Short Papers
The mating behaviour
of Atlantic cod (Gadus morhua)
Sherrylynn
Rowe and Jeffrey A. Hutchings
Department of Biology, Dalhousie University, Halifax, Nova Scotia, B3H
4J1, Canada. rowes@is2.dal.ca
Introduction
Atlantic cod (Gadus morhua) is a marine demersal fish that inhabits cool-temperate to subarctic waters from inshore regions to the edge of the continental shelf on both sides of the North Atlantic (Scott and Scott 1988). Atlantic cod has been harvested throughout its range for hundreds of years and yet despite being of theoretical interest and practical importance, very little has been learned about its reproductive behaviour during this period.
Throughout the Atlantic, there are many recognized cod stocks, each of which has its own set of characteristics. Age at maturity varies between 2 and 7 years (Myers et al. 1997) and Atlantic cod typically spawn over a period of less than 3 months (Brander 1994; Chambers and Waiwood 1996; Kjesbu et al. 1996) in water depth ranging from tens (Smedbol and Wroblewski 1997) to hundreds of metres (Brander 1994; Morgan et al. 1997). Individuals are assumed to breed annually and Atlantic cod are considered to be batch spawners as only 5-25% of a female’s egg complement is released at any time during her 3- to 6-week spawning period (Chambers and Waiwood 1996; Kjesbu et al. 1996). Individual females release hundreds of thousands, often millions, of tiny eggs (1.2-1.6 mm in diameter), for which no parental care is provided, directly into oceanic waters (Scott and Scott 1988).
The limited information available on Atlantic cod spawning behaviour suggests complex mating patterns, the occurrence of behavioural and acoustic displays by males, mate choice by females, and alternative reproductive strategies among males (Brawn 1961a; Hutchings et al. 1999). However, there is no information on the selective causes and consequences of these behaviours, nor the structure of the mating system (Nordeide and Folstad 2000).
Our research employs a quantitative approach to understand causes and consequences of variation in the mating system of Atlantic cod at the individual and population levels. We are incorporating both detailed experimental studies in the laboratory and observations of cod in the wild. Our research involves several components including the following: (i) mating system structure and identification of behavioural and phenotypic correlates of reproductive success, (ii) intra- and inter-population variation in sound production during spawning, and (iii) patterns of variation in drumming muscle mass.
Laboratory observations of spawning Atlantic cod
The laboratory component of our research involves cod from two spatially distinct areas in the Northwest Atlantic: Southwest Scotian Shelf and Southern Gulf of St. Lawrence, identified by the Northwest Atlantic Fishery Organization (NAFO) Divisions as 4X and 4T, respectively. Mature adults from each stock were collected and taken to a 680 m3 aquarium at Dalhousie where spawning occurred. Groups of fish representing each stock were examined separately during their temporally distinct spawning periods. Cod were maintained at densities similar to those in nature (approximately 0.1 fish per m3; Rose 1993; Morgan et al. 1997) and spawning behaviour of individually tagged fish was recorded by videotape and visual observation. A hydrophone was placed in the centre of the tank and sounds were recorded continuously during the spawning season. A random sample of fertilized eggs was collected daily and pedigree analysis is being undertaken using microsatellite DNA. In addition to providing information on individual male and female reproductive success, the DNA analyses, coupled with behavioural observations, will allow us to determine phenotypic and behavioural correlates of reproductive success.
Observations to date suggest that strong differences in spawning behaviour exist between and within Atlantic cod populations. A strong dominance hierarchy, territoriality, and high levels of aggression characterized males from 4T but this wasn’t the case for males from 4X among which very little aggressive behaviour occurred and no fish held territories for prolonged periods. However, preliminary examination of the sound recordings have suggested that 4X fish are more vocal than 4T fish. Similarly, we found that for both females and males, 4X fish had heavier drumming muscles relative to their body weight (drumming muscle somatic index) than those from 4T (Figure 1).
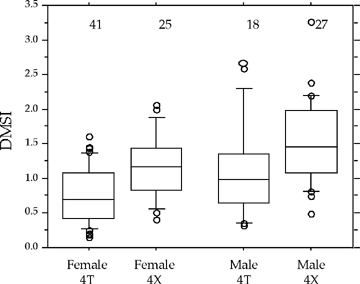 |
| Figure 1. Drumming muscle somatic index (DMSI) for female and male Atlantic cod from Northwest Atlantic Fishery Organization (NAFO) Divisions 4T and 4X. Box plots indicate outliers (points) and 10th, 25th, 50th, 75th, and 90th percentiles. Sample sizes given above estimates. |
We also observed intra-population differences in behaviour, particularly for fish from 4T. We found that the aquarium was dominated by 3-4 males that fiercely defended territories during the spawning season and frequently engaged in courtship activity. Other males were much more passive and it seems that some specialized as sneakers in spawning events and even might have been imitating females to gain access to male territories. Initial observations suggest that fish which were dominant and engaged in most courtship activity were ones with the largest drumming muscle somatic index (and were not necessarily the largest in length). We are waiting for results of the pedigree analysis to determine the way in which these behaviours might have influenced reproductive success.
Field observations of variation in Atlantic cod drumming muscle mass
Sound production by males is hypothesized to be important to successful mating in cod (Brawn 1961a, b; Engen and Folstad 1999; Hutchings et al. 1999) and we wanted to examine patterns of variation in the size of their sound-producing "drumming" muscles in more detail. Brawn (1961b) found that Atlantic cod produced sound most frequently during the spawning period and although both sexes were capable of producing sounds throughout the year, only males seemed to do so during the spawning season, typically during aggressive defense of territories and courtship display.
During March 2001 - February 2002, we sampled approximately 100 cod/month from NAFO Division 4X to quantify seasonal and individual variation in body condition and drumming muscle mass. We found that drumming muscle mass tended to increase with fish size. Furthermore, when we controlled for fish size, we found that spawning males had larger drumming muscles than non-spawning males (Figure 2) but there was no such relationship for females. In addition, males had larger drumming muscles than females both during spawning and non-spawning seasons.
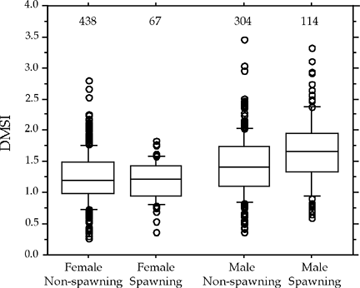 |
| Figure 2. Drumming muscle somatic index (DMSI) for non-spawning and spawning female and male Atlantic cod from Northwest Atlantic Fishery Organization (NAFO) Division 4X. Box plots indicate outliers (points) and 10th, 25th, 50th, 75th, and 90th percentiles. Sample sizes given above estimates. |
Interestingly, we also observed a weak, but significant relationship between drumming muscle somatic index and body condition for males in spawning condition (Figure 3). This suggests that male drumming ability could convey reliable information to females about mate quality.
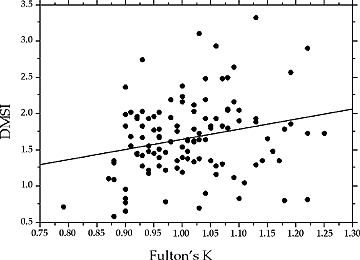 |
|
Figure 3. The relationship between drumming muscle somatic index (DMSI) and body condition (as indicated by Fulton’s K condition factor) for spawning male Atlantic cod from Northwest Atlantic Fishery Organization (NAFO) Division 4X. |
Conclusions
We will continue our research on Atlantic cod by combining pedigree data with phenotypic and behavioural observations of fish in our aquarium to assess correlates of reproductive success. Also, we will study sound production by cod in more detail by examining the types and characteristics of sounds produced, the behavioural contexts in which sounds occur, and temporal patterns of sound production.
In recent years, stock collapses have caused many Atlantic cod fisheries to be reduced and others even closed. Knowledge of Atlantic cod spawning behaviour will likely contribute to better understanding of population dynamics and improved ability to predict the effects of fishing on cod populations.
Acknowledgements
Thanks to Jim Eddington, Paty Avendano, and the Fishermen and Scientists Research Society for technical assistance. Financial support for this research was provided by a Natural Sciences and Engineering Research Council grant and a Petro-Canada Young Innovator award to JAH. We are also grateful for financial support for travel provided by the sponsors of the International Workshop on the Application of Passive Acoustics in Fisheries.
References
Brander, K. 1994. Spawning and life history information for North Atlantic cod stocks. ICES Coop. Res. Rep. No. 205.
Brawn, V.M. 1961a. Reproductive behaviour of the cod (Gadus callarias L.). Behaviour 18: 177-198.
Brawn, V.M. 1961b. Sound production by the cod (Gadus callarias L.). Behaviour 18: 239-255.
Chambers, R.C., and K.G. Waiwood. 1996. Maternal and seasonal differences in egg sizes and spawning characteristics of captive Atlantic cod, Gadus morhua. Can. J. Fish. Aquat. Sci. 53: 1986-2003.
Engen, F., and I. Folstad. 1999. Cod courtship song: a song at the expense of dance? Can. J. Zool. 77: 542-550.
Hutchings, J.A., T.D. Bishop, and C.R. McGregor-Shaw. 1999. Spawning behaviour of Atlantic cod, Gadus morhua: evidence of mate competition and mate choice in a broadcast spawner. Can. J. Fish. Aquat. Sci. 56: 97-104.
Kjesbu, O.S., P. Solemdal, P. Bratland, and M. Fonn. 1996. Variation in annual egg production in individual captive Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 53: 610-620.
Morgan, M.J., E.M. DeBlois, and G.A. Rose. 1997. An observation on the reaction of Atlantic cod (Gadus morhua) in a spawning shoal to bottom trawling. Can. J. Fish. Aquat. Sci. 54: 217-223.
Myers, R.A., G. Mertz, and S. Fowlow. 1997. The maximum population growth rates and recovery times of Atlantic cod (Gadus morhua). Fish. Bull. U.S. 95: 762-772.
Nordeide, J.T., and I. Folstad. 2000. Is cod lekking or a promiscuous group spawner? Fish and Fisheries 1: 90:93.
Rose, G.A. 1993. Cod spawning on a migration highway in the north-west Atlantic. Nature 366: 458-461.
Scott, W.B., and M.G. Scott. 1988. Atlantic Fishes of Canada. Can. Bull. Fish. Aquat. Sci. 219. 731 pp.
Smedbol, R.K., and J.S. Wroblewski. 1997. Evidence for inshore spawning of northern Atlantic cod (Gadus morhua) in Trinity Bay, Newfoundland, 1991-1993. Can. J. Fish. Aquat. Sci. 54: 177-186.