|
Singapore-MIT Alliance for Research & Technology |
BioSystems and Micromechanics (BioSyM) Inter-Disciplinary Research Group |
||||||||||
|
|
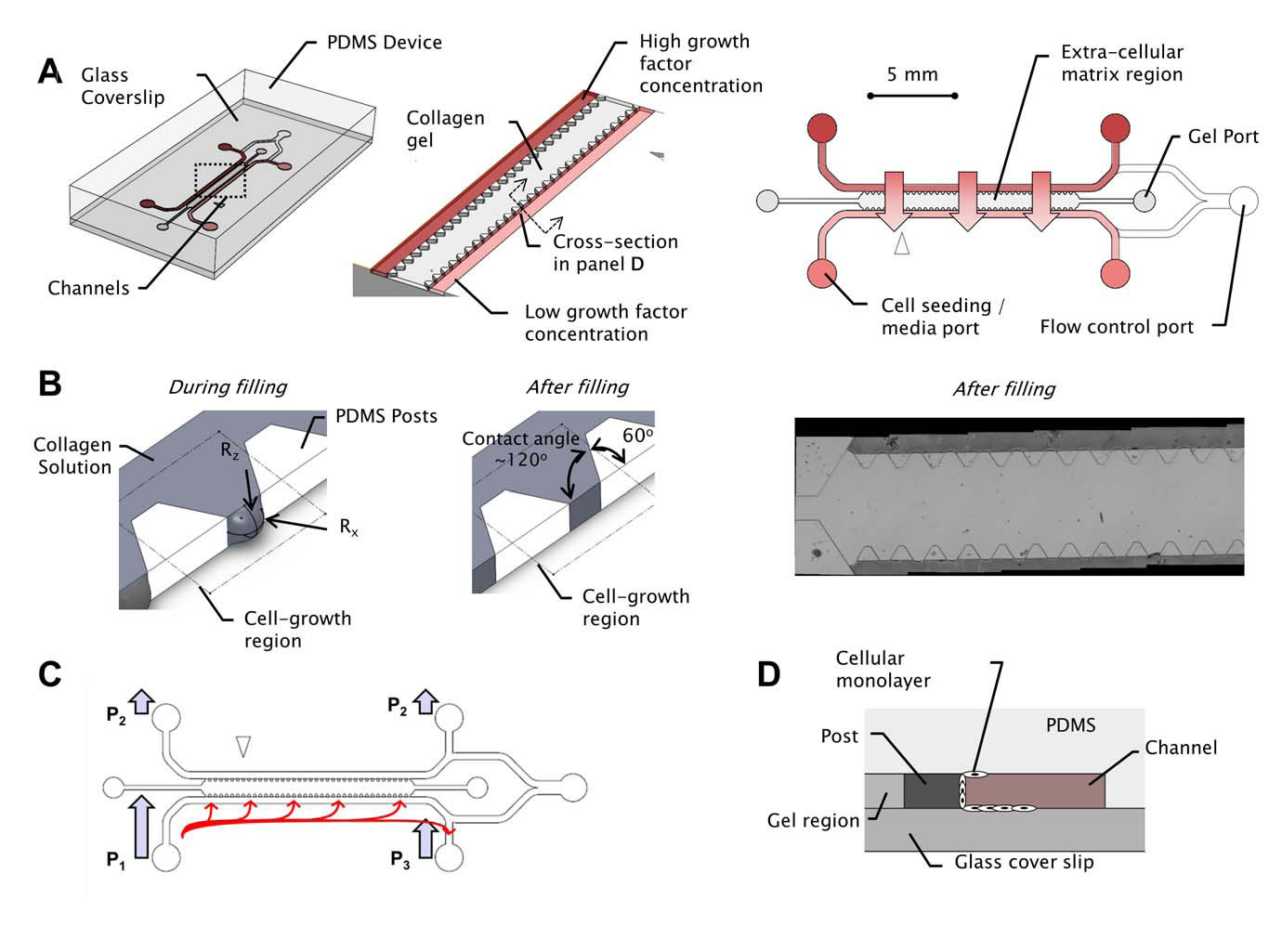
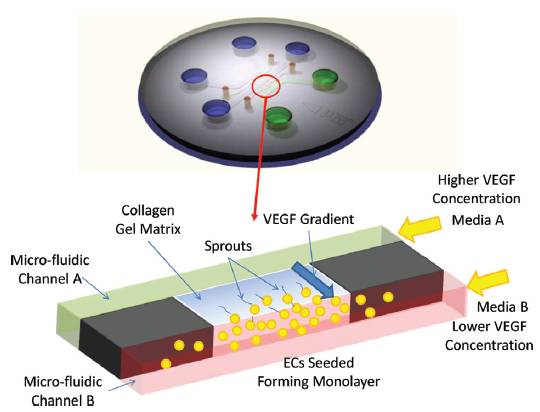
BioSyM ResearchThrust 3: In vitro cellular systems engineeringResearch Projects(Harry Asada (SMART-BioSyM), Roger Kamm (MIT/SMART-BioSyM), Ong L.Sharon (SMART-BioSyM), Min Cheol Kim (SMART-BioSyM), Waleed Farhat (MIT), Levi Wood (MIT), Alisha Schor (MIT) and Devin Niel (MIT)The use of microfluidic cell cultures to characterize the response of multi-cellular systems in vitro has gained traction in the experimental study of a variety of biological processes, including angiogenesis, neuronal growth and development, immune cell responses and micro-tissue cultures among many others. We have studied ensemble three-dimensional cell cultures and quantitative analysis of angiogenic growth from uniform endothelial monolayers. Our approach combines two key elements: a micro-fluidic assay that enables parallelized angiogenic growth instances subject to common extracellular conditions, and an automated image acquisition and processing scheme enabling high-throughput, unbiased quantification of angiogenic growth. Because of the increased throughput of the assay in comparison to existing three-dimensional morphogenic assays, statistical properties of angiogenic growth can be reliably estimated...........Our findings are consistent with stochastic agent-based mathematical models of angiogenesis that represent angiogenic growth as a series of independent stochastic cell-level decisions.
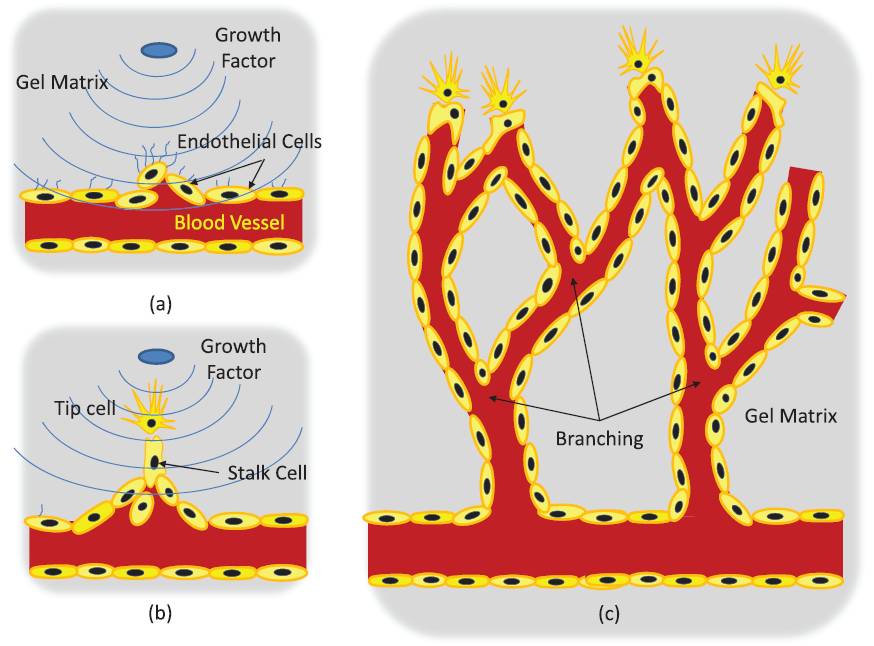
2. Control of sprout development in angiogenesis(Harry Asada (SMART-BioSyM), Roger Kamm (MIT/SMART-BioSyM), Ong L.Sharon (SMART-BioSyM), Min Cheol Kim (SMART-BioSyM), Waleed Farhat (MIT), Levi Wood (MIT), Alisha Schor (MIT) and Devin Niel (MIT)Angiogenesis is the process of generating a vascular network from an existing blood vessel. A population of Endothelial Cells (ECs) residing in a blood vessel can sprout out and create a new vascular network when exposed to growth factors. Cells communicate with each other and interact with the matrix field in a stochastic manner, leading to pattern formation as a result of collective cell behaviors.
Our goal is to establish feedback control to reduce variance in angiogenic development and form desired vascular geometries and patterns. We have developed an open-loop map between system cues (exogenous inputs) and angiogenic response over 2-4 days and automated confocal image processing methods to evaluate the data. We have proposed models and control algorithms to control sprout elongation rate by modulating the chemotactic gradient. Combining these relationships will be essential to providing a closed loop control scheme that regulates sprout geometry. 3. Spatio-Temporal Image Analysis of Cell Sprouting with Bayesian Estimation(Ong L.Sharon (SMART-BioSyM), Harry Asada (SMART-BioSyM), Ang Marcelo (NUS)The project goal is to develop an automated analysis of the growth of endothelial cell sprouts in in-vitro experimentation using time-lapse and fixed images from confocal microscopy to aid the understanding of cell behaviors and interactions. We approach these multi-cell tracking challenges via probabilistic methodologies. A Kalman filtering combined with Multiple Hypothesis Testing (MHT) and smoothing/retrodiction is proposed to allow tracking of varying cell dynamics and account for clutter due to close contact cells. In addition to that, probabilistic techniques are used to incorporate fixed end-point imaging data with time-lapse information in a mathematically consistent manner. 4. Manipulation of Stiffness Gradients in Extracellular Microenvironment through Stochastic Control of Magnetic-Particle Ensemble(Peter Chen (NUS), Harry Asada (SMART-BioSyM), Sahan Christie Bandara Herath (NUS)The mechanical properties of microstructures that surround cells play an important role in determining the behavior of a cell population, including differentiation, proliferation, and apoptosis. The objective of this project is to develop engineering approaches to directly manipulate the extracellular microenvironment in order to produce desired changes in its stiffness. 5. Prolyl Hydroxylases-inhibitors Induced Angiogenesis in a Microfluidic Device(Roger Kamm (MIT/SMART-BioSyM), Michael Raghunath (NUS) and Lim Sei Hien (NUS/SMART-BioSyM)Angiogenesis has been implicated in more than 70 disorders so far such as the ischemic heart disease, respiratory distress and etc. Consequently, various approaches to overcome these diseases have been proposed, including transfected cells expressing angiogenic peptides, local delivery of angiogenic peptides and many others. Nonetheless, those approaches are less preferred as they deal with one or few angiogenic factors at a time which is different from the physiological conditions where the angiogenesis is regulated by at least 20 angiogenic growth factors such as VEGF, placental growth factor, interleukin-8 and etc while many others are yet to be identified. The main goal of this project is to study the angiogenic effect of PHi (prolyl hydroxylase inhibitors) on HUVEC cells in a defined way where the concentration gradient of growth factors can be established while the angiogenesis could be observed in a 3D scaffold which is a step closer to the in vivo condition. 6. Three-Dimensional Microfluidic Bioassay for Early Differentiation of Murine Embryonic Stem Cells(Roger Kamm (MIT/SMART-BioSyM) and Young Kum Park (SMART-BioSyM)Embryonic stem cells cultured in a three-dimensional collagen gel scaffold within a microfluidic device were investigated by live confocal microscopic imaging and IMARIS analysis. Using an efficient three-dimensional microfluidic bioassay, we qualitatively and quantitatively determine the differentiation ratio of embryonic stem cells based on the expression of a vascular progenitor cell marker, Flk-1. 7. In Vitro Preclinical Microfluidic Screening for Drug-Like Molecules as Cardiomyocyte Differentiation Agents(Roger Kamm (MIT/SMART-BioSyM), Mayasari Lim (NTU), Young Kum Park (SMART-BioSyM), Ong L.Sharon (SMART-BioSyM), Se Young Yang (MIT) and Devin Niel (MIT)There is increasing importance in the study of screening drug-like small molecules to induce cardiomyocyte differentiation from ES cells by way of a robust and dependable bioassay. However, an efficient and cost-effective in vitro screening for this investigation has not been successfully developed yet. Here, we focus on the development of an ES cell-based microfluidic device with multi-parametric control, high-throughput, and time-lapse imaging for screening drug-like target molecules on cardiomyocyte differentiation. 8. Mesenchymal Stem Cells paracrine contributions on vascular formation and stabilization(Jerry Chan (NUS-Duke/KKH), Roger Kamm (MIT/SMART-BioSyM) and Liu Yuchun (NUS)MSC paracrine activity stimulates repair at the wound site through release of angiogenic factors. Here, we aim to investigate the effect of these factors present in MSC conditioned media on EC behaviour in vitro in a microfluidic platform, identify the critical factors that stimulate angiogenesis, followed by ischemic rescue upon bolus delivery of MSC conditioned media in vivo in an ischaemic animal study model. Here we (a) investigate the effect of MSC conditioned media (MSCCM) culture on EC growth, proliferation and survival in vitro; (b) identify the angiogenic proteins present in MSCCM through a proteomic approach.; (c) study the migratory behaviour of EC as well as vascular stability/(disintegration) upon MSCCM treatment in the microfluidic devices over extended durations under real-time imaging; (d) isolate angiogenic proteins for use as treatment groups in microfluidic devices to study their involvement in vascular formation/stabilization and thus, identify critical angiogenic factors; (e) investigate the effect of in vivo injection of bolus MSCCM/ isolated factors on limb recovery in a hindlimb ischaemic animal model.
|
|
|||||||||