
Research
| Hydrogen Bond Dynamics in Water | ||
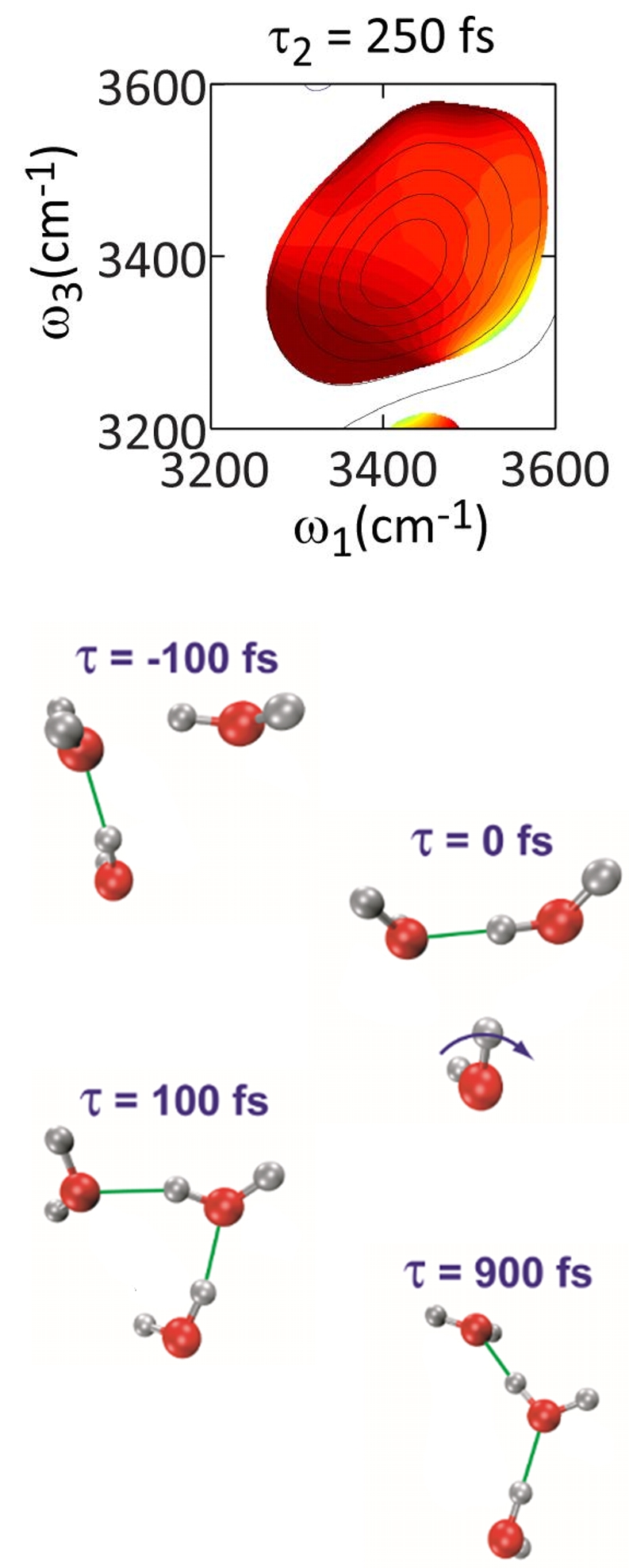
Water is composed of an ever-changing network of hydrogen bonds, whose structural evolution underlies its unusual physical properties, its important role in guiding aqueous chemistry and charge transport, and its role in hydrophobicity and biological folding and self-assembly. Our group has used 2D IR spectroscopy and other ultrafast nonlinear infrared spectroscopies to monitor the fluctuations of this network and build a mechanistic picture of how water’s structure evolves with time. The OH frequency of dilute HOD dissolved in D2O is sensitive to the hydrogen bonding configuration at the proton since the formation of a hydrogen bond tugs on the intermolecular potential and lowers the OH frequency. By tracking this frequency over time we gain insight into how water's fastest intermolecular motions act to break and form hydrogen bonds. We complement our experiments with spectroscopic modeling that draws on molecular dynamics simulations. We have used these methods to demonstrate that hydrogen bonds in water are broken only transiently during the exchange of hydrogen bonding partners. This rearrangement involves the concerted motion of multiple water molecules, and proceeds through large angle reorientation through a birfurcated hydrogen bond transition state. Representative Publications “Structural Rearrangements in Water Viewed Through Two-Dimensional Infrared Spectroscopy,” S. T. Roberts, K. Ramasesha, and A. Tokmakoff, Accounts of Chemical Research 42 1239-1249 (2009) . “Collective Hydrogen Bond Reorganization in Water Studied with Temperature-Dependent Ultrafast Infrared Spectroscopy,” R. A. Nicodemus, S. A. Corcelli, J. L. Skinner, and A. Tokmakoff, Journal of Physical Chemistry B, 115 5604-5616 (2011). “Ultrafast 2D IR Anisotropy of Water Reveals Reorientation during Hydrogen-Bond Switching,” K. Ramasesha, S. T. Roberts, R. A. Nicodemus, A. Mandal and A. Tokmakoff, Journal of Chemical Physics, 135 054509 (2011). |
 |
|
Proton transfer in aqueous hydroxide solutions |
||
|
Compared to ions of similar size and charge density, the aqueous hydroxide ion possesses an anomalously fast diffusion constant due to its ability to accept a proton from a neighboring water molecule, leading to the translocation of the ion. While this mechanism is generally recognized, a description of the OH- solvation structure and mechanism of proton transfer to the ion remains controversial. Ultrafast infrared spectroscopy is particularly useful for the study of proton transport mechanism, since the OH spectroscopy directly reflects the local proton potential. We perform 2D IR experiments to assign the spectral features to the OH- ion and its solvation shell, and to characterize the kinetics of exchange between these species. We observe a spectral feature that decays on a ~110 fs time scale that we assign to the relaxation of transiently formed HO..H..OH– species, where a proton is equally shared between a HOD molecule and an OD- ion. Over picosecond waiting times, we observe the exchange of population between OH- ions and HOD molecules due to deuteron transfer giving a lower bound of 3 ps for the deuteron transfer kinetics. “Observation of a Zundel-like transition state during proton transfer in aqueous hydroxide solutions,” Sean T. Roberts, Poul B. Petersen, Krupa Ramasesha, Andrei Tokmakoff, Ivan S. Ufimtsev, and Todd J. Martinez, Proceedings of the National Academy of Sciences, USA 106 15154-15159 (2009) . “Proton Transfer in Concentrated Aqueous Hydroxide Visualized using Ultrafast Infrared Spectroscopy,” Sean T. Roberts, Krupa Ramasesha, Poul B. Petersen, Aritra Mandal, and Andrei Tokmakoff, Journal of Physical Chemistry A 115 3957-3972 (2011). |
|