Research
| Introduction | |
|
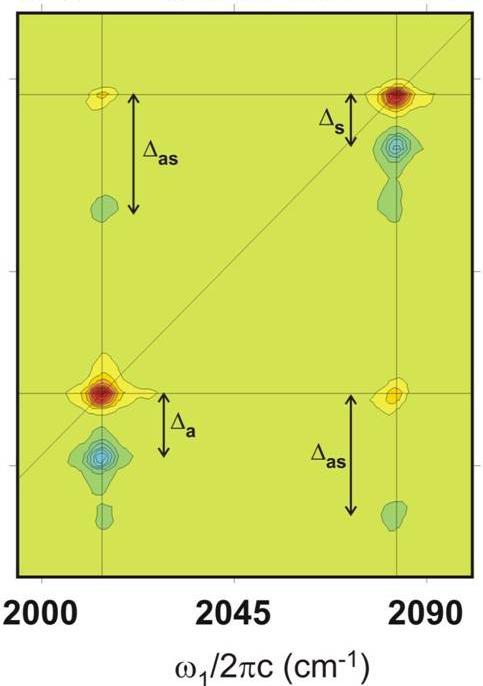
The Tokmakoff group uses ultrafast vibrational spectroscopy to study the molecular dynamics of chemical and biological processes in solution. We are interested in revealing the time-scale and mechanism by which the hydrogen bond network of water rearranges; how the dynamical nature of water's hydrogen bonds influence processes such as proton transfer and hydrophobicity; the time-scale and mechanism of conformational change by proteins, peptides, and DNA in folding, recognition, and binding. These questions all involve understanding the molecular details of competing non-covalent interactions effects, such as repulsion, electrostatics, and hydrogen bonding. We try to deduce which internal coordinates are relevant to the dynamics and how these are probed by our experiments, to build broadly applicable models for these processes. Our experimental approach involves development of new structurally sensitive femtosecond vibrational spectroscopies that can be used to follow the time-evolution of molecular structure. The most sensitive and broadly applicable tool is two-dimensional infrared spectroscopy, which we use to capture information on transient molecular structure and characterize structural variation. Experiments are complemented by theoretical work on nonlinear spectroscopy, statistical mechanical modeling of dynamics and relaxation, and molecular dynamics computer simulations. Our research is supported by the Department of Energy and the National Science Foundation. For further details you can read about: |