10.213 Lecture Notes
Mass Balances
Optional reading at the Reserve Book Room, 14N-132:
MASS BALANCES
Mass is conserved by almost every chemical process encountered by chemical engineers. The only exceptions are processes in which nuclear reactions occur, such as in a nuclear power plant.
Despite their simplicity, mass balances are extremely useful for analyzing chemical processes. Whole areas of equipment design are based on mass balances and a few other relationships.
SETTING UP AND USING A MASS
BALANCE
rate of accumulation = rate of input - rate of output + rate of generation
The rates of accumulation and generation involve changes occurring inside the envelope. The rates of input and output concern the flows crossing the envelope boundaries.
The rate of generation is associated with chemical reactions occurring inside the mass balance envelope. This rate is positive for net species production and negative for net species destruction. In the absence of chemical reactions inside the mass balance envelope involving the chosen chemical species, the rate of generation term is identically zero, a significant simplification. Since inerts, elements (i.e. carbon atoms), and certain sums of reaction and product species satisfy this last criterion, these species are often desirable for mass balance calculations.
The rate of accumulation accounts for the buildup or depletion of species within a piece of equipment when it is operated in a transient (i.e. non-steady state) mode. For instance, consider a sink with a completely closed drain. As water is run from the faucet there is a positive rate of accumulation indicating a buildup of water in the sink. Conversely if the faucet is shut off and the drain is opened, the loss of water from the sink can be accounted for by a negative rate of accumulation. If the rate of water addition from the faucet exactly matches the rate at which water drains, the rate of accumulation is zero. That is the amount of water in the sink remains fixed and the system is said to be at steady state.
Most chemical processes are assumed to run at steady state, which implies that there are no variations of the process varies with time. Thus, rate of accumulation is identical zero under the condition of steady state.
Thus, for a non-reacting species at steady state, the mass balance equation for each species reduces to:
rate of input = rate of output
This is an incredibly simple concept. However, the application of this concept may or may not be so simple depending on what needs to be calculated and how the problem is formulated.
There will be N+1 possible mass balance equations that describe a system containing N different chemical species. Of these equations, N are associated with one of the N species and a final equation is a balance on the total mass of the system. In the absence of chemical reaction, only N of these equations are independent. Any possible set of N equations can be utilized to solve for the unknown variables. The one equation which was left out will not provide any additional information. If there are Nrxn independent chemical reactions occurring in the system, the number of independent mass balances equals N - Nrxn, as each reaction provides an additional constraint on the system.
EXAMPLE PROBLEM
(paraphrased from Hougen (ref. 2 above), pg. 230, problem #5)
Steady-state with no chemical reaction
Spent acid from a nitrating process contains 21% H2SO4, 55% HNO3 and 24% H2O by weight. This stream is to strengthened by the addition of
1) concentrated sulfuric acid containing 93% H2SO4 and 7% H2O
and
2) concentrated nitric acid containing 90% HNO3 and 10% H2O
to form a stream whose composition is 62% H2SO4, 28% HNO3 and 10% H2O.
If 1000 lbs/hour of the product stream is desired, find the flow rates of the other three streams.
For any mass balance problem, certain information will be given and the unknown information will be constrained by the number of independent mass balances relationships that exist. When the number of unknown variables is exactly equal to the number of mass balance constraint the problem has been properly designed and all the unknowns can be determined, as in example on the previous page. In contrast, underdefined problems have too many unknowns and usually has an infinite number of solutions while overdefined problems have too few unknowns and often have no solution.
By determining the degrees of freedom (DOG) associated with a given mass balance envelope, we can determine if the problem is properly set up. In addition, we can determine which choice of a mass balance envelope will be easiest to solve when multiple choices are possible. For any mass balance envelope:
DOF = {total # of independent variables} - {# of independent specified variables and relationships} - {the # of independent mass balances}
Lets consider the right-hand side of this equation term by term using the mass balance envelope of the previous example:
The first term represents the total number of independent variables needed to specify the flow rate and composition of each inlet and outlet stream to the mass balance envelope. For a single stream, M independent variables are needed if M species are present. Summing over all the inlets and outlet streams gives the total # of independent variables for entire mass balance envelope. Referring to the previous example, the M values for inlet streams are 3 for A, 2 for B, and 2 for C. Adding these to M=3 for the product stream yields a total of 10 independent variables for this mass balance envelope.
The second term requires counting up the total number of independent pieces of information that are given in the problem. Since problem statements vary in the way in which information is presented, take care not to include dependent information.
One of the simplest formats is when only the flow rates of an individual species are counted. As an example, consider the product stream in the example above. In this case, M=3, and all three mass flow rates of individual species are given, totally specifying this stream without the need for any unknowns associated with this stream.
Alternatively, total flow rate and M-1 fractions can be picked. So an equivalent description of the product stream would be a total flow of 1000 lbs/hr having weight fractions of 0.62 H2SO4 and 0.28 HNO3. Note that the last fraction, that of the water, is a dependent variable and can be obtained by subtracting the sum of all the other fractions from one. Continuing this analysis for the other streams shows that for inlets B and C only one independent weight fraction is given, while two independent fractions are given for A. Adding the 4 independent specification associated with the inlet streams to the 3 associated with the product stream, accounts for a total of 7 independent variables in this problem are unspecified. These three can be taken as the total flow rates, A, B, or C of the three inlet streams, which we would like to find.
Sometimes information is given neither as the flowrates of individual components nor as compositional fractions. For example, the problem could state the ratio of two components in a given stream is limited to a certain value. This relationship is an additional piece of information which should also be included in the second term of the DOF equation if it is independent of the other information given. In other words, this relationship can not be derived solely from other constraints of the problem. for instance, consider a two component stream. If we are told the mole ratio of two components is 4 to 1, this counts as an independent relationship if no other compositional information is given. However if we are also given that the mole fraction of one component is 0.8, the mole ratio could be derived from this information and provides no additional information.
The final term on the right hand side of the DOF equation has been discussed
above, and is simply equal to N-Nrxn, where
N is the total number of different chemical species.
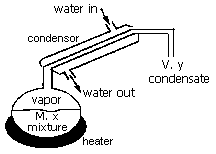
Example of mass balance on a transient system with no chemical reaction
BATCH DISTILLATION
A flask contains a mixture including component A. It is desired to produce
a condensed vapor which is enriched in component A as compared to the liquid
mixture contained in the flask by using the apparatus below:
 |
Variables
|
First, consider the general mass balance equation
Accumulation = Input - Output + Generation (1)
If the total number of moles in the mass balance envelope is considered, two of the terms in this equation are zero. Since no material is being added and there are no chemical reactions, the simplified relationship is:
Expressing these terms as function of the defined variables yields:
Now, the mass balance on component A can be considered, equation (2) still applies,
d(Mx)/dt = -y·V (4)
Using the product rule to expand the derivative and multiplying by dt yields:
x dM + M dx = -y·V dt (5)
Of the three variable in this equation, one can be eliminated using equation (3):
x dM + M dx = y dM (6)
rearranging gives Rayleigh's equation for batch distillation:
In order to be able to solve this equation, a vapor-liquid equilibrium
relationship between the x and y is needed. An example of a VLE relationship
is Henry's law, y=Kx. Often, VLE relationships
are more mathematically complex. Upon substitution into equation
(7) and integration, the variation in the degree of enrichment of the
vapor in A as the mass in the flask decreases can be found.
Example of mass balance on a multiple unit process with chemical reaction
BUTANE ISOMERIZATION
Normal butane (n-C4) is being isomerized to isobutane (i-C4) in a reactor for use in the manufacture of isooctane. It is desirable to convert a very high fraction of the n-C4 fed to the process, but the reaction does not go to a high degree of completion in one pass through the reactor. As a result, the products from the reactor are separated in a distillation column into an overhead product which is very rich in i-C4 and a bottom product which is rich in n-C4. the overhead product is removed from the process. The bottom product is recycled to join with the pure n-C4 feed to the process; this mixture is fed to the reactor.
The steady-state feed (F) to the process is one mole per minute of pure n-C4 and a ratio of i-C4 to n-C4 equal to one is desired for the reactor effluent (E). The overhead product (P) from the distillation column is 99 mole% i-C4 and the reactor is designed to convert a 9:1 mixture of n-C4 to i-C4 to a 1:1 mixture in a single pass.
Sketch the process showing the reactor, the distillation column, the process feed, the process product stream, and the recycle stream. Calculate the total steady-state flow rates of the product (P) and recycle (R) streams. What is the value of x, which is the mole fraction of i-C4 in stream R?