EXAMPLE PROBLEM
(paraphrased from Hougen (ref. 2 above), pg. 230, problem #5)
Steady-state with no chemical reaction
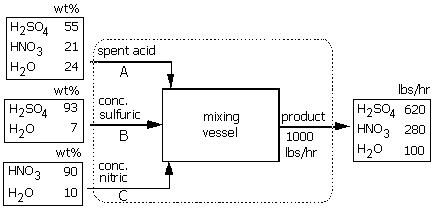
Spent acid from a nitrating process contains 21% H2SO4, 55% HNO3 and 24% H2O by weight. This stream is to strengthened by the addition of
1) concentrated sulfuric acid containing 93% H2SO4 and 7% H2O
and
2) concentrated nitric acid containing 90% HNO3 and 10% H2O
to form a stream whose composition is 62% H2SO4, 28% HNO3 and 10% H2O.
If 1000 lbs/hour of the product stream is desired,
find the flow rates of the other three streams.
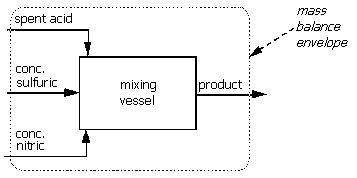
1) Need a sketch of the problem which has been stated in words. Should show all inlet (waste acid, concentrated sulfuric acid and concentrated nitric acid) and outlet (product) streams and the mixing vessel.
2) Draw the mass balance envelope. Since there is only one piece of equipment in the process, there is only one reasonable choice.

3) Label all the streams. The compositions of all inlet and outlet flows are given in addition to the flow rate of the product stream. The unknown flow rates of the inlet stream are named as variables A, B and C, with units of lbs/hour.
4) Choose a basis for calculations. Since no chemica reactions are present, the masses of H2SO4, HNO3 and H2O can be used directly. A flowrate basis of 1000 lbs/hour of product stream will be chosen, since this will directly yield the numbers asked for the problem statement directly.
5) The process is at steady-state and involves no chemical reaction, so the equation
rate of input = rate of output
can be applied to each of the N species to obtain
3 independent equations
| H2SO4 | 0.55A + | 0.93B | = 620 | |
| HNO3 | 0.21A + | 0.90C | = 280 | |
| H2O | 0.24A + | 0.07B + | 0.10C | = 100 |
Solving gives A=126.8 lbs/hr, B=591.6 lbs/hr, and C=281.6 lbs/hr. Simple!
Note that the fourth possible mass balance equation on the total flows, A+B+C = 1000 lbs/hours, is satisfied as expected for this dependent relationship and provides no additional information. That is the total mass in equals that out, as expected at steady state.