

|
|
| Thermodynamics and Propulsion | |
Subsections
3.4 Refrigerators and Heat Pumps
The Carnot cycle has been used for power, but we can also run it in
reverse. If so, there is now net work into the system and net heat
out of the system. There will be a quantity of heat
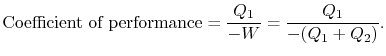
For a Carnot cycle we know the ratios of heat in to heat out when the cycle is run forward and, since the cycle is reversible, these ratios are the same when the cycle is run in reverse. The coefficient of performance is thus given in terms of the absolute temperatures as

This can be much larger than unity. The Carnot cycles that have been drawn are based on ideal gas behavior. For different working media, however, they will look different. We will see an example when we discuss two-phase situations. What is the same whatever the medium is the efficiency for all Carnot cycles operating between the same two temperatures.
3.4.0.1 Refrigerator HardwareTypically the thermodynamic system in a refrigerator analysis will be a working fluid, a refrigerant, that circulates around a loop, as shown in Figure 3.7. The internal energy (and temperature) of the refrigerant is alternately raised and lowered by the devices in the loop. The working fluid is colder than the refrigerator air at one point and hotter than the surroundings at another point. Thus heat will flow in the appropriate direction, as shown by the two arrows in the heat exchangers.
Starting in the upper right hand corner of the diagram, we describe the process in more detail. First the refrigerant passes through a small turbine or through an expansion valve. In these devices, work is done by the refrigerant so its internal energy is lowered to a point where the temperature of the refrigerant is lower than that of the air in the refrigerator. A heat exchanger is used to transfer energy from the inside of the refrigerator to the cold refrigerant. This lowers the internal energy of the inside and raises the internal energy of the refrigerant. Then a pump or compressor is used to do work on the refrigerant, adding additional energy to it and thus further raising its internal energy. Electrical energy is used to drive the pump or compressor. The internal energy of the refrigerant is raised to a point where its temperature is hotter than the temperature of the surroundings. The refrigerant is then passed through a heat exchanger (often coils at the back of the refrigerator) so that energy is transferred from the refrigerant to the surroundings. As a result, the internal energy of the refrigerant is reduced and the internal energy of the surroundings is increased. It is at this point where the internal energy of the contents of the refrigerator and the energy used to drive the compressor or pump are transferred to the surroundings. The refrigerant then continues on to the turbine or expansion valve, repeating the cycle. Douglas Quattrochi 2006-08-06 |