The Dincă Lab focuses on the synthesis and characterization of new inorganic and organic materials for applications in small molecule transformations (e.g. CO2 and O2 reduction, and natural gas conversion), energy conversion and storage, sensing, gas separation, and biotechnology. By designing and synthesizing new materials, we also hope to learn more about fundamental processes such as electron and ion transport through ordered solids, the reactivity and electrochemistry of low-coordinate metal ions in porous crystals, the effects of conformational changes on the electronic properties of molecules, and the behavior of materials at the interface with solid-state devices and biological media.
The Synthesis of Conductive MOFs for Applications in Energy Research
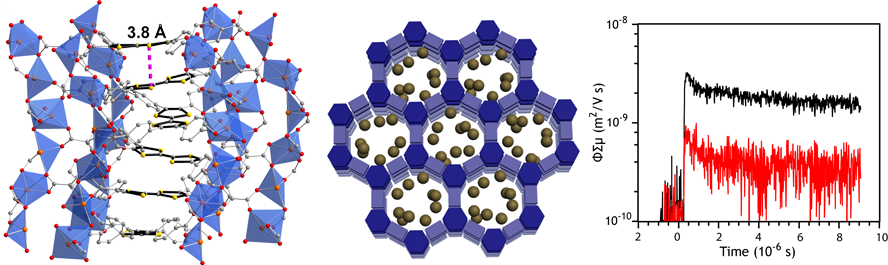
Metal-organic frameworks have traditionally been used for gas storage and separation, and much less attention has been devoted to their electronic properties. We are synthesizing new metal-organic frameworks (MOFs) that exhibit good charge mobility and conductivity. We have recently shown that the incorporation of tetrathiafulvalene moieties into a zinc-based metal-organic framework can produce solids with an intrinsic charge mobility of 0.2 cm2/Vs (above, left). This is the highest mobility ever observed in a MOF and is commensurate with the bulk charge mobilities of the best conducting polymers used in organic photovoltaics. Our group is also actively exploring methods to engender electronic conductivity in a related class of materials called covalent-organic frameworks, and more generally, porous electroactive polymers. COFs have inherent π-stacked two-dimensional structures that can lend themselves to hole, electron, and potential one-dimensional ion mobility naturally (above, middle). We are also exploring systems with redox-active metals and softer ligands that form bonds with greater covalency and π-overlap with the metal orbitals. Students involved in this project design and synthesize new ligands, synthesize the targeted MOFs and COFs using solvothermal reactions, and measure physical properties with sophisticated techniques available either in house or through collaborators in the US or abroad (above, right). Our development of conductive high-surface area materials could provide unique new architectures for organic photovoltaics, new membranes for rechargeable Li- and Na-ion batteries, new materials for potential-swing separation, and electrocatalysts for CO2 and O2 reduction.
Small Molecule Activation and the Synthesis of Redox-Active MOFs
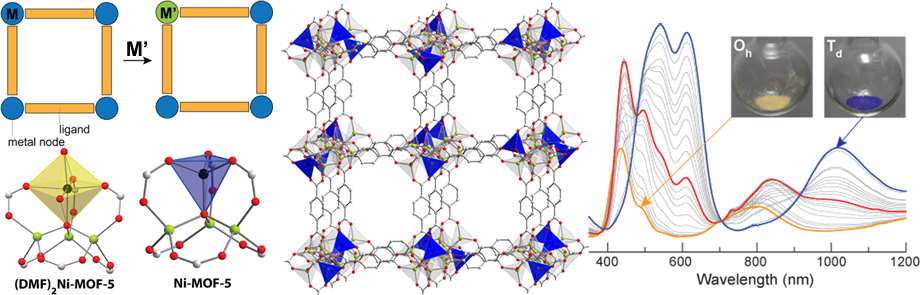
Similar to zeolites, metal-organic frameworks could function as veritable solid-state scaffolds for a variety of small molecule transformations relevant to chemical feedstocks and energy conversion. We aim to synthesize new ligands and materials that will take advantage of the inherently rigid nature of MOFs and introduce redox-active metal centers with particularly unusual and reactive coordination spheres. One particularly promising and efficient method to turn MOFs that show no redox reactivity into exciting redox-active materials is ion exchange. We have shown, for instance, that Zn2+ ions in Zn4O(1,4-benzenedicarboxylate)3 (MOF-5), arguably the most famous material in its class, can be substituted with a variety of other metal ions (above), thereby making MOF-5 redox-active and amenable for small molecule activation. We are now exploring the scope of the post-synthetic ion metathesis (PSIM) approach, aiming to understand the fundamentals of this very broad and incredibly useful technique. Ultimately, we wish to explore the reactivity of the highly unusual metal centers that we can isolate within MOFs either directly or via PSIM. These materials could serve as efficient catalysts for a series of transformations of industrial importance. Currently, we are interested in using natural gas as a chemical feedstock and are studying new oxidation chemistry with redox-active MOFs. Students involved in this project become familiar with ligand synthesis and solvothermal techniques, gas sorption studies, and a variety of in-situ spectroscopy techniques that are crucial for characterizing the materials and their reactivity towards molecules such as O2, NO, and N2O.
Turn-On Porous Sensors: the Effect of Conformation on the Electronic Properties of Molecules
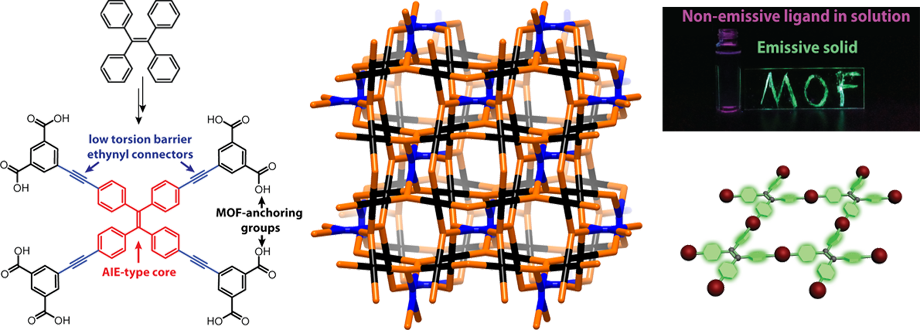
Owing to their highly crystalline nature, metal-organic frameworks (MOFS) and covalent-organic frameworks (COFs) can function as perfect scaffolds for controlling the collective properties of electronically non-trivial organic molecules or metal clusters. They are therefore excellent platforms for studying the formation and propagation of excitons. We have also begun taking advantage of the rigidity of MOFs to isolate organic molecules such as tetraphenylethylene (TPE, above) in unusual conformations to study the effect of conformation on photophysics. The ultimate aim is to make turn-on porous sensors for gases, pressure, and viscosity, for instance, by using ligands based on a class of chromophores known as aggregation-induced emission (AIE) chromophores, whose iconic representative is TPE itself. We have shown that TPE forms porous fluorescent MOFs despite being itself non-lfuorescent, and used spectroscopic techniques to derive rules for making turn-on porous sensors using these molecules. Students involved in this project often design and synthesize relatively complicated organic molecules, become profficient in solvothermal synthesis, and a variety of spectroscopic techniques such as fluorescence specotroscopy and solid-state NMR, done in collaboration with the Griffin group. Theoretical calculations also inform our synthetic design. More widely, we are interested in using topological principles to control through novel synthesis the aggregation sequence of molecular chromophores, with potential applications in solar energy conversion and light harvesting constructs.
Thin Films of MOFs for Gas Separation Membranes and CO2 Reduction Electrocatalysis
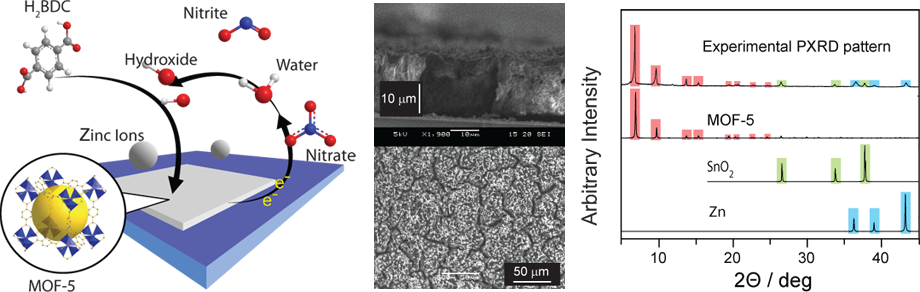
Deposition of MOFs as thin films and membranes - Current synthetic methods are not useful for the deposition of MOFs directly and selectively on conductive surfaces, as needed for instance for the use of MOFs as electrocatalysts. More generally, the lack of good methods for making thin films and membranes prevents the use of these promising materials in practical settings and is a key requirement for future developments. We are devising new methods that afford the synthesis and deposition of MOF thin films, membranes, and nanoparticles directly on conductive surfaces, through electrochemistry. We have shown recently that tens of microns-thick films of MOF-5 can be deposited selectively (from among a host of other possible products) from a solution containing Zn2+ and terephthalic acid in only minutes at room temperature. This contrasts with typical solvothermal techniques, which require high temperature and extended time. The method also allows phase-selection via potential control. We are currently exploring the scope of our electrodeposition approach, and the effect of variables such as deposition potential, electrode surface, concentration, and electrolyte. Developing conformal film growth methods, as allowed by electrochemistry, will lead us to MOF-based membranes for gas separations, which are currently some of the most energy-intensive processes in the world.
CO2 reduction using MOF thin films - Owing to the generally insulating nature of MOFs, these have not been used almost at all for electrocatalysis applications. As such, thin films are needed to explore their potential to function as electrocatalysts. Enabled in part by our MOF thin-film deposition method described above, we are studying CO2 electroreduction uisng MOFs. The use of CO2 as a feedstock for value-added chemicals is an important target for sustainability. Students involved in these projects will become experts in electrochemical and solvothermal techniques and will be involved in organic synthesis, as necessary for designing and synthesizing new ligands. We also aim to develop soft, solution methods that would enable facile patterning of various solid-state materials on the underlying surface, which is very challenging using current strategies.
