Fourier phase microscopy for investigating live
cell structure and dynamics
| Investigators: |
G. Popescu, T. Ikeda, M.S. Feld |
Optical microscopy has been the most commonly used method of investigating
live cells and various related technologies have been developed
over the past years [1]. Various biological samples, including live
cells, are quite transparent under visible light illumination and
behave essentially as phase objects. Techniques like phase contrast
and Nomarski/ DIC microscopy couple the sample phase information
into the intensity distribution and are capable of reveling structural
details of biological systems. However, with these instruments,
the information about the phase shift associated with the illuminating
field is only qualitative. A few years ago, pioneering experiments
performed in our laboratory demonstrated that retrieving the phase
information from biological structures in a quantitative manner
allow for a variety of novel applications in the biological investigation
of structure and dynamics [2]. Both interferometric [3, 4] and non-interferometric
[5] techniques have been proposed for quantitative phase imaging
of biological samples. However, a microscope capable of delivering
quantitative phase images with sub-nanometer sensitivity over extended
periods of time (comparable to a cells life cycle) is still highly
desirable.
The LCI Group at the Spectroscopy Laboratory has recently developed
a highly sensitive phase imaging instrument, referred to as the
Fourier Phase Microscope (FPM), which relies on the principle of
decomposing a given field into its average and a spatially varying
field [6]. Thus the average field plays the role of the reference
field, as in a typical interferometric setup. In our setup, the
two interfering fields are derived by Fourier transforming the output
image of an existing transmission microscope, which provides excellent
transverse resolution and long term stability.
FPM results on standard samples
In order to demonstrate the ability of FPM to retrieve phase images
in a quantitative manner, we performed experiments on various calibrated
phase objects, such as phase gratings and polystyrene beads. Figure
1 shows an example of such measurements on 3 micron beads immersed
in glycerol. The transmission intensity image is shown in Fig. 1a.
The contrast of this image is poor, due to the transparency of the
sample. Figure 1b shows the phase contrast image. Although this
image shows greatly improved contrast, it cannot provide the thickness
of the sample. The FPM image (Fig. 1c) exhibits high contrast and
provides quantitative information about the sample thickness. To
assess the stability of the instrument against phase noise and,
thus, quantify its sensitivity to optical path length changes, a
cell chamber containing water only (no particles) was continuously
imaged at intervals of 15s. Figure 1d shows the temporal optical
path length fluctuations associated with a point contained in the
field of view over a 100 minute period; the CCD exposure time was
50 ms for each interferogram. The standard deviation of these fluctuations
had a value of 0.15nm, which is equivalent to 1/5,500 of a wavelength.
This result demonstrates the remarkable path length sensitivity
of the FQPM and its potential for investigating long term time-varying
processes. The extremely low noise is due to the fact that the two
interfering fields traverse a common optical path. In addition,
the Fourier processing is performed on a magnified microscope image
of the sample, which further narrows the optical path of the interfering
fields and also provides improved mechanical stability. The use
of a low-coherence illumination field, as opposed to laser radiation,
contributes to the sensitivity of the method, as fringes created
by multiple reflections on various components are suppressed.
 |
| |
FPM images on live cells
The FPM was further applied to image live cells. HeLa cells (an
epithelial cell line derived from a cervical neoplasm) were continuously
monitored over periods of up to 12 hours at repetition rates of
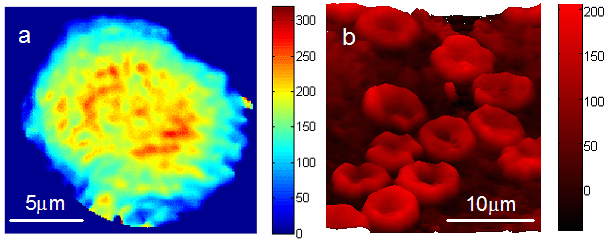
4 frames/ minute. Figure 2a shows the color-coded quantitative phase
image of a cell during the metaphase of mitosis, revealing the structure
of separating chromatids. The cells were imaged in typical culture
conditions (i.e. immersed in culture medium) and no preparation
was performed prior to the measurement. Therefore, quantitative
phase images can be reconstructed over extended observation periods,
allowing quantitative analysis of cellular dynamics such as shape
change or growth. We note that the quantitative phase information
is also suitable for automatic cell motility analysis. Figure 2b
shows the pseudo-color quantitative phase image of a whole blood
smear. The sample was prepared by sandwiching a small drop of fresh
blood between two cover slips. The well-known discoid shape of red
blood cells is recovered. Simple analysis that takes into account
the refractive index of hemoglobin with respect to plasma can easily
provide information about the volume of red blood cells. The FPM
can therefore provide a high-throughput procedure for screening
various abnormalities in red cells and potentially other blood constituents.
Summary
In summary, the FQPM provides a non-perturbative means of accurately
measuring phase images of cells and other biological structures
in their natural states, without sample pre-processing. Its high
stability allows for time-varying processes to be studied over periods
of many hours. Studies in our laboratory are exploring its potential
in various aspects of cell biology..
Recent Publications
- D. J. Stephens and V. J. Allan, "Light microscopy techniques
for live cells imaging." Science, 300,
82 (2003).
- C. Yang et al., Optics Letters., Interferometric phase-dispersion
microscopy, 20, 1526 (2000); C. Yang et al., Phase dispersion
optical tomography, Opt. Let., 26, 686 (2001); C. Yang et al.,
Phase-referenced interferometer with subwavelength and subhertz
sensitivity applied to the study of cell membrane dynamics, Opt.
Lett., 26, 1271 (2001).
- G. A. Dunn and D. Zicha, Using DRIMAPS system of transmission
interference microscopy to study cell behavior, in Cell biology:
a laboratory handbook, 2nd Ed , J. Celis, (Academic press1997),
pp.44.
- H. Kadono, M. Ogusu, and S. Toyooka, Phase shifting common
path interferometer using liquid-crystal phase modulator, Opt.
Comm, 110, 391-400 (1994).
- D. Paganin and K. A. Nugent, Noninterferometric phase imaging
with partially coherent light, Phys. Rev. Lett., 80, 2586 (1998).
- G. Popescu, L. P. Deflores, K. Badizadegan, J. C. Vaughan,
H. Iwai, R. R. Dasari, and M. S. Feld, Fourier phase microscopy
for investigation of biological structure and dynamics, Opt. Lett.
in press (2004).

|
 |