Introduction
Definition of Life
Motivation
Preliminary
Steps
Present Life
Past Life
Geological Survey
Sample Collection
Spectroscopic Analysis
Organic Analysis
Biology Experiments
Thin Section
Isotope Analysis
Experimental Design
Pyrolytic Release Experiment
Brief Synopsis
The pyrolytic release experiment (PR) is based on the assumption
that terrestrial life synthesizes organic material from carbon dioxide (CO2)
and carbon monoxide (CO). This
experiment called for the incubation of a soil sample with radioactively
marked CO2 and CO and the subsequent measurement of radioactive particles
in the soil afterward. This
experiment also produced unexpected results, which proved to conclude that
either there was no life or if there were life, it did not assimilate the
CO2 and CO. Once again,
making more restrictions on the sample type tested could solve the main
problems of this experiment.
Equipment
- Viking composite instrument including 14C detector
- CO2 labeled with 14C
- Source of krypton to pressurize the chamber
- Heat source
- Light source (xenon arc lamp)
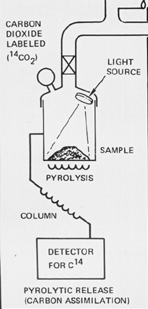
Protocol
n place .25 cc of soil in the soil chamber
n add 20mL of radioactively labeled CO2 and CO
n add krypton to pressurize the chamber
n incubate the sample under xenon arc lamp for 120 hours (5 days)
n flush sample of atmosphere and heat to 635°C to pyrolyze any organic material
n run organic material (gases) through 14C detector
The incubation under the lamp is to maximize the assimilation of the radioactive carbon compounds into any organic material of the soil. The wavelength of the xenon lamp was constrained to over 320nm to ensure the absence of any nonbiological, photocatalyzed synthesis of organic compounds from the CO and CO2.
Viking results
The data show that a fixation of atmospheric carbon occurs in the surface material of Mars under conditions approximating the Martian ones. Although the amount of material was small relative to that which organisms on Earth produced, it was still more significant than the margin of error.
Problems
Due to the lack of organic material as detected by the GC/MS, the probability of there existing such organic material was slim and made the results hard to believe. In addition, the argument was brought up that any Martian organisms would have been killed under the lamp energy as it provided heat that greatly exceeded its normal climate while the sample tested positive after being heated. Therefore, the material detected must have been the product of the nonbiological reactions. Other alternate explanations for the results include the carry-over of any particulate matter from the incubation chamber to the column between the chamber and the 14C detector and the incorporation of the radioactively marked CO into a carbon polymer that existed on the Martian soil. Another explanation is related the existence of peroxides and superoxides; the CO could have been reduced by H2O2 to produce the organic material that the detector was testing for.
Alterations made to protocol
While this experimental protocol is more conservative than others, it still needs to be narrowed down to specific types of soil. Basically, the conditions for the soil are the exact same as the conditions mentioned for the labeled release experiment. By imposing those conditions, we can successfully eliminate most of the problems and uncertainties. Furthermore, we can test the suggested soil sample to ensure that no other inorganic molecule such as H2O2 and the carbon polymer interfere with the successful assimilation of the carbon.
Sources
Levin, Gilbert V. and Patricia Ann Straat, “Recent Results From the Viking Labeled Release Experiment on Mars.” Journal of Geophysical Research. Vol. 82, No. 28 September 30, 1977
Carr, Dr. Michael H. and Benton Clark et al. “An
Exobiology Strategy for Mars Exploration.” Online: http://cmex.arc.nasa.gov/MarsNews/mars_papers/Docs/mars_strat.html
NASA, “Science on Mars.” Online: http://history.nasa.gov/SP-4212/ch11-5.html
Caplinger, Michael. “Life on Mars.” Online: http://barsoom.msss.com/http/ps/life/life.html
For a handy semi-animation of this experiment, go to http://cybooks.com/ahill3/hill03.html