Project Amazonia: Monitoring - Modeling
Population viability analysis (PVA) is a process of identifying the threats faced by a species and evaluating the likelihood that it will persist for a given time into the future.
Population viability analysis is often oriented towards the conservation and management of rare and threatened species, with the goal of applying the principles of population ecology to improve their chances of survival. Threatened species management has two broad objectives. The short term objective is to minimize the risk of extinction. The longer term objective is to promote conditions in which species retain their potential for evolutionary change without intensive management. Within this context, PVA may be used to address three aspects of threatened species management:
- Planning research and data collection. PVA may reveal that population viability is insensitive to particular parameters. Research may be guided by targeting factors that may have an important impact on extinction probabilities or on the rank order of management options.
- Assessing vulnerability. Together with cultural priorities, economic imperatives and taxonomic uniqueness, PVA may be used to set policy and priorities for allocating scarce conservation resources.
- Ranking management options. PVA may be used to predict the likely response of species to reintroduction, captive breeding, prescribed burning, weed control, habitat rehabilitation, or different designs for nature reserves or corridor networks.
Population Viability Analysis is a technique for determining the probability that a species will become extinct within any given time period. It is applied almost exclusively to mammals, although it is occasionally used to evaluate the status of birds. PVA has the unique ability to take into account a great deal of subtle information about a species, including the age-specific birth and death rates, the ratio of males to females at birth, the negative effects of inbreeding on small populations, and even randomly occurring calamities, such as floods and climatological events. Since many aspects of PVA models are randomly determined, a model is normally run many times in order to establish the statistical properties of the results. Thus, a common output of these analyses is a histogram of Time to Extinction, from which it is possible to determine the probability that the species will have gone extinct at a given time from present.
Population Viability Analyses are not normally possible without information on the present state of the population. Thus, monitoring must begin before PVA can be done. However, once accurate data is available, PVA can show which species are in danger in the long term. This is not necessarily the same as those species which appear endangered in the short term; in fact, a very large population at any one time might indicate more danger for a species than a moderate population size, due to the population exceeding the carrying capacity of the ecosystem. PVA can forecast such population crashes.
By providing information about which species are truly in danger, PVA also allows better allocation of resources for monitoring and species- specific preservation efforts. PVA turns experimental data from monitoring animal species into useful information about the future.
We must keep in mind that there is an enormous amount of factors that contribute to the health of the ecosystem. However, for coming up with a hypothetical index number, we will consider the biomass because the amount of biomass is a direct consequence of all the biotic and abiotic factors.
To address this need, the study quantified the total aboveground biomass (TAGB) and forest structure in tropical forest sites in Brazil. The TAGB of intact forests range from 288 to 346 Mg ha-1, with a mean of 313 Mg ha-1; dense forests TAGB range from 298 to 533 Mg ha-1, with a mean of 377 Mg ha-1; and ecotone forests TAGB range from 298 to 422 Mg ha-1, with a mean of 350 Mg ha-1. In general, the mean TAGB is 341 Mg ha-1. Non-tree components comprise 22% of TAGB. This is noteworthy because the non-tree components are often omitted from forest biomass/carbon pool estimates.
Information on total aboveground biomass (TAGB) is scarce for Amazonian forests. Indirect estimates based on commercial volume from forest inventory data1, as well as direct field measurements of individual trees have been used to predict TAGB2. Estimates for TAGB in the Brazilian Amazon have ranged from 155 to 555 Mg ha-1.
TAGB will be estimated by measuring all organic materials above mineral soil. TAGB will be divided into "tree" (broad-leaved trees) and "non-tree" (other components, predominantly palms) components based on structural and ecological significance and practicality of measurement.
Tree diameter will be measured at 1.37 m above the ground (dbh). Trees will be separated into seven diameter classes based on dbh (<10, 10-30, 30-50, 50-70, 70-100, 100-200 and >200 cm dbh). Palms will be sampled separately from broadleaf trees. We will divide them into three categories (basal palms with no trunks, <10 and 10 cm dbh). Vines and lianas will be placed in two size classes (<10 and 10 cm dbh). Other components include small dicots (plants <1.37 m in height), litter/rootmat (forest floor), standing dead trees and palms, and dead and downed coarse woody debris (CWD).We will divide CWD into two categories: 2.5-7.6 and 7.6 cm diameter3. The forest floor component is composed of litter, small wood debris (<2.5 cm diameter), and rootmat. Rootmat contains a large amount of decomposing organic matter, as well as live roots.
Individual equations for each forest component will be used for calculating the
biomass (Table 1 and Table 2). Biomass of trees 5 cm dbh will be calculated from
equations based on dbh given by Higuchi et al. (1998) for Amazonian trees.
Biomass of trees <5 cm dbh will be calculated from equations based on dbh given
by Hughes et al. (1999). Tree height will be estimated from a regression
equation with tree diameter as the independent variable.
Steps involved with the methodology
Biomass of CWD will be calculated by using the methods of Van Wagner (1968). Transects to measure mass of CWD 7.6 cm in diameter were 15 m long. Pieces of CWD that are 2.5-7.6 cm in diameter will be measured along the central 5 m of the 15-m transect. Coarse woody debris will be further separated into tree (dicot) wood or palm wood components. The 7.6 cm diameter class will also be separated into sound or rotten classes following the methods of Kauffman et al. (1988) and Brown (1971).
For the 2.5-7.6 cm diameter classes, the diameter and angle off the horizontal of 65 individual pieces along a 100 m transect will be measured to calculate the quadratic mean diameter and wood particle tilt5. Thereafter, we will only count pieces that intersect the line, and we used density, quadratic mean diameter, and wood particle tilt variables to calculate biomass.
To calculate forest floor biomass, each sample will initially be weighed in the field. Sub-samples will then be oven-dried to determine the ratio of wet-to-dry weight. This ratio will then be applied to the entire sample to convert from wet-to-dry weight.
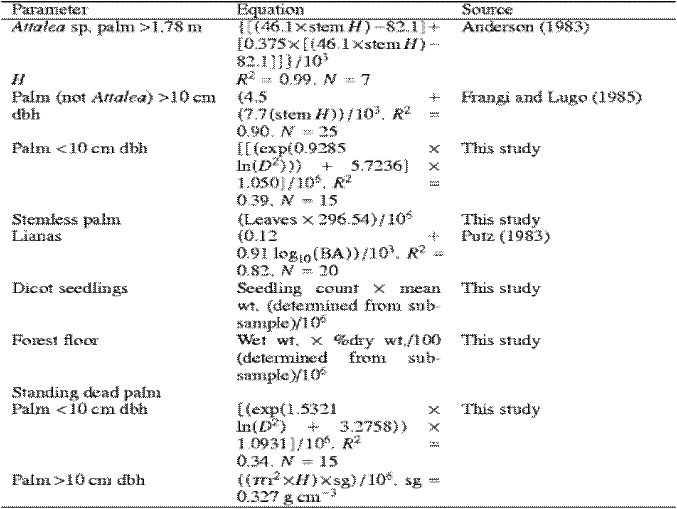
To estimate biomass of basal leaf palms, the number of leaves of each individual palm encountered in the 2 m×10 m plot will be counted and multiplied by a mean weight per leaf derived from a random sample of 30 basal leaves that will have been oven-dried and weighed. Three equations will be necessary to ascertain biomass of palms: biomass of Attlea sp. 1.78 m high will be calculated with the model developed by Anderson (1983); biomass of other palm species 10 cm dbh will be estimated with the model of Frangi and Lugo (1985); and biomass of palms <10 cm dbh will be calculated by using a model developed specifically for this study.
Vine biomass estimates will be calculated with the model given by Putz (1983). All seedlings (<1.37 m height) will be counted in each of the 16 (1 m×1 m) plots per site. Seedling biomass will be based on sub-sample of 50 randomly collected oven-dried seedlings from which an average weight per seedling will have been determined.
Biomass of standing dead trees <10 cm dbh will be calculated from an equation developed by Hughes et al. (1999). Biomass of standing dead trees 10 cm dbh will be estimated by first calculating volume, then multiplying volume by the mean value of specific gravity of sound dead wood. Standing dead palm biomass will be estimated from an equation developed for this study for palms <10 cm dbh and by multiplying volume by specific gravity (0.327 g cm-3) for palms 10 cm dbh.
Table 1: Individual equations for each forest component that are used for calculating the biomass4
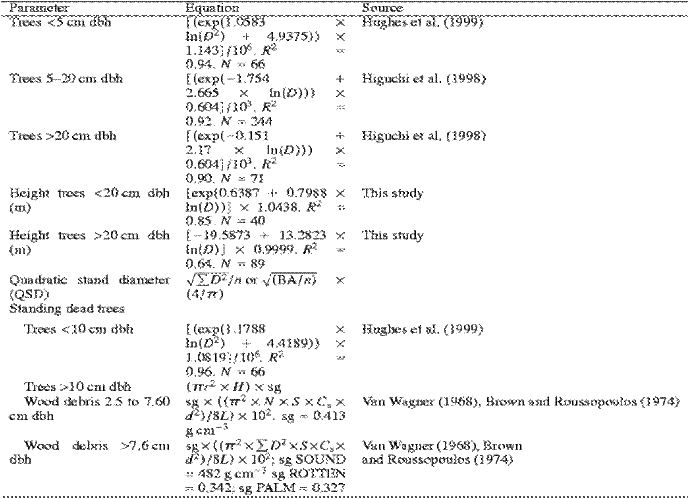
Table 2: Individual equations for each forest component that are used for calculating the biomass4
Biological Value
According to the National Research Council, there are five basic criteria that researchers look at when determining the biological value of a given species or habitat5:
1. Richness
- the number of species or habitat in a given area. A region with more species
per unit area is given a higher value.
Ex. Tropical forests have higher conservation priority than an adjacent
tropical dry forest with less richness of species.
2. Endemism - the narrowness of distribution of species in an area. A
region with many endemic species has a higher value than a region with fewer
endemic species. Endemics are the species that are prevalent in or peculiar to
an area.
Ex. Madagascar which has 80% of plant species found nowhere else in the
world has high priority.
3. Rarity - the rarity of species in a region. A region with rare species
is given a higher value.
4. Ecosystem Services - The importance of the natural habitat or resident
single species capable of influencing ecosystem function for various services of
importance to humans.
Ex. Forested
watershed that is a source of public water has higher conservation value.
5. Protected Status - The relative protection of species that already
exists determines the value of a species or habitat.
All in all, species and habitats do not all have equal value when
it comes to biodiversity management and conservation, and we must come up with
the species and habitats that will best represent the health of the Amazon
rainforest as a whole.
End of Monitoring
![]()
1: Brown, S. and Lugo, A.E., 1984. Biomass of tropical forests: a new estimate based on forest volumes. Science 223, pp. 1290-1293. Abstract-GEOBASE
Brown, S., Lugo, A.E., 1990. Biomass estimates for Brazil's Amazonian moist forests. In: Forest'90: Annals of the First International Symposium on Environmental Studies on Tropical Rain Forests, Manaus, Brazil, pp. 46-52.
Brown, S. and Lugo, A.E., 1992. Aboveground biomass estimates for tropical moist forests of the Brazilian Amazon. Interciencia 17, pp. 8-18.
Brown, S., Lugo, A.E. and Iverson, L.R., 1992. Processes and lands for sequestering carbon in the tropical forest landscape. Water Air Soil Pollut. 64, pp. 139-155. Abstract-Compendex | Abstract-GEOBASE | Abstract-EMBASE
2: Jordan, C. and Uhl, C., 1978. Biomass of a terra firme forest of the Amazon Basin. Oecologia Plantarum 13, pp. 387-400.
Klinge, H. and Rodriguez, W., 1973. Biomass estimation in central Amazonian rain forest. Acta Cient. Venez. 24, pp. 225-237.
Klinge, H., Rodriguez, W., 1974. Phytomass estimation in a central Amazonian rain forest. In: Proceedings of the IUFRO Congress on Forest Biomass Studies, Vol. 15, Rome.
3: Kauffman, J.B., Sanford, R.L., Cummings, D.L., Salcedo, I.H. and Sampaio, E.V.S.B., 1994. Biomass and nutrient dynamics associated with slash fires in neotropical dry forests. Ecology 74, pp. 140-151.
Kauffman, J.B., Cummings, D.L., Ward, D.E. and Babbit, R., 1995. Fire in the Brazilian Amazon. I. Biomass, nutrient pools, and losses in slashed primary forests. Oecologia 104, pp. 397, 408. Abstract-Elsevier BIOBASE | Abstract-GEOBASE
4: Brown, J.K., 1974. Handbook for Inventorying Downed Woody Material. USDA Forest Service, Ogden, UT, pp. 25.
Brown, S., 1997. Estimating Biomass and Biomass Change of Tropical Forests: A Primer. Forestry Paper 134, FAO, Rome.
5: National Research Council, et al., 1999. Perspectives on Biodiversity: Valuing its Role in an Everchanging World. Washington, D.C.: National Academy Press.
