  |
|
Welcome to Mission 2016
The Future of Strategic Resources
Solving Complex Problems (12.000) is designed to provide students the opportunity to work as part of a team to propose solutions to a complex or “unsolveable” problem that requires a strongly interdisciplinary approach. Over the past four years we have focused on large problems related to the environmental health and sustainability of the planet.....from collapse of the global fisheries, to access to clean fresh water, to stemming the rise of greenhouse gases, to feeding nine billion people. A common theme is that almost all proposed solutions will cost enormous amounts of money and thus we will be forced to prioritize! This year we will focus on strategic natural resources.
 The global distribution of strategic and economically vital metals is highly variable with some highly developed countries rapidly exploiting and controlling much of the supply. Much new technology is fueled by rare metals such as lithium, which is used in batteries, tantalum in mobile phones, iridium and germanium in computers. New technologies such as hybrid cars and wind turbines use neodymium, a Rare Earth Element. China is now the number one producer of Rare Earths and controls the market. In 2010, China cut off exports of REE’s to Japan in retaliation for a dispute over territorial waters. This in turn caused the U.S. Congress to push the Department of Energy to seek new sources of REE’s which are essential to both military and commercial uses. Global “Green” technologies (electromagnets and batteries) are using REE’s in ever increasing amounts. For example a Prius electric motor requires two pounds of neodymium and twenty two to thirty three pounds of Lanthinum for its battery! This raises a more general question of what are environmental and economic trade-off’s on “Green” technology. The global distribution of strategic and economically vital metals is highly variable with some highly developed countries rapidly exploiting and controlling much of the supply. Much new technology is fueled by rare metals such as lithium, which is used in batteries, tantalum in mobile phones, iridium and germanium in computers. New technologies such as hybrid cars and wind turbines use neodymium, a Rare Earth Element. China is now the number one producer of Rare Earths and controls the market. In 2010, China cut off exports of REE’s to Japan in retaliation for a dispute over territorial waters. This in turn caused the U.S. Congress to push the Department of Energy to seek new sources of REE’s which are essential to both military and commercial uses. Global “Green” technologies (electromagnets and batteries) are using REE’s in ever increasing amounts. For example a Prius electric motor requires two pounds of neodymium and twenty two to thirty three pounds of Lanthinum for its battery! This raises a more general question of what are environmental and economic trade-off’s on “Green” technology.
Other critical minerals include the platinum-group metals (PGEs) used in catalytic converters, key to reducing air pollution. Both the U.S. and the EU are very concerned about future supplies of a number of elements that include in addition to the REEs and PGEs, gallium, indium, niobium, and tantalum. The EU also includes metals used in alloys such as antimony, berrilium, cobalt, germanium, and tungsten. Projected shortages are starting a race for financial interests in new sources from newly discovered ores to the seafloor to outer space. Only a few nations have the financial capabilities to do this while others worry about feeding their people. Should we allow the rapid exploitation of global supplies by and for the developed nations or can we develop a more equitable approach? The future of the planet very much depends on this. 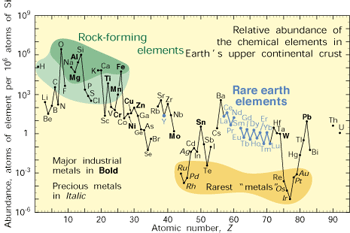 In addition, an element we take for granted, phosphorus, which is essential as fertilizer for feeding the world, is rapidly being exploited, with some predicting extreme shortages by the end of the next century. The U.S. imports phosphorus while China does not export it. As the human population expands to more than 7 billion and 9 billion by 2050, the demand for all of these resources is going to rise. Shortages raise the possibility of international strife, and even war, over non equitable distribution and demand of limited resources. In addition, an element we take for granted, phosphorus, which is essential as fertilizer for feeding the world, is rapidly being exploited, with some predicting extreme shortages by the end of the next century. The U.S. imports phosphorus while China does not export it. As the human population expands to more than 7 billion and 9 billion by 2050, the demand for all of these resources is going to rise. Shortages raise the possibility of international strife, and even war, over non equitable distribution and demand of limited resources. Your Mission is to devise plans to ensure that all nations, including those that aspire to be developed, have access to these ever decreasing resources by implementing recycling technologies, searching for non-traditional sources, and developing an environmentally sensitive global management plan. Mission 2016 is also part of the Terrascope program and the issues associated with the future of strategic natural resources, the year-long theme of Terrascope. By enrolling in 12.000 you become part of the Terrascope program and community, even if you do not continue in the Spring. See and hear what former students are saying about the program here. Spring Field Trip:During spring break in March of 2013 we will take a field trip to explore the problems and issues associated with the Mission 2016 theme. In the past, the location has involved international travel but ultimately depends on the directions you take in class and the nature of your solution. We will keep you updated. About 12.000:"Solving Complex Problems" (12.000) is a nine-unit, Fall-semester subject designed to provide freshmen with the opportunity to work as part of an interdisciplinatry team to design a viable solution to a complex problem. This year it will be known as Mission 2016 - The Future of Strategic National Resources.Each year's class explores a different problem in detail through the study of complimentary case histories and the development of creative solution strategies. It includes developing a website, literature surveys, and development of creative solution strategies. Initially developed with major financial support from the Alex and Britt d'Arbeloff Fund for Excellence in MIT Education, and now supported through Dean for Undergraduate Education (DUE), 12.000 is designed to enhance the freshman experience by helping students develop contexts for other subjects in the sciences and humanities, and by helping them to establish learning communities that include upperclassmen, faculty, MIT alumni, and professionals in science and engineering fields. 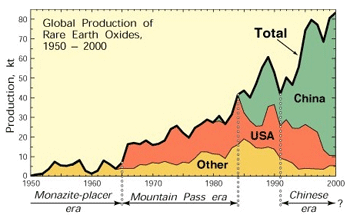 Why Mission?The Mission class offers freshmen a completely different way to learn. In contrast to the core classes that rely on lectures and problem sets, Mission attempts to teach students how to think about solving complex problems. Students in Mission are independent, largely self-directed, and interactive. They learn how to build teams and develop solutions that require teamwork between scientists and engineers including economics and social sciences. Mission students will learn that many problems are just too big and complex to be solved by any one person or discipline and must involve integration. At the end of the class the students of Mission will have developed new and innovative solutions to an "unsolvable" problem and been exposed to a variety of different disciplines. Unlike any other class open to freshmen, we treat you as capable science and engineering researchers from Day 1 and our expectation is for you to produce a plan that will attract the attention of people from around the world. Websites developed for past Missions still elicit questions and comments from around the world years after they were posted.  History of the ClassMission, or 12.000, was offered first in Fall 2000, when the assignment (Mission 2004) was to develop a viable mission plan for the exploration of Mars with the aim of finding evidence for the present or past existence of life. The assignment for Fall 2001 (Mission 2005) was to design undersea research stations for both coral reef and abyssal environments. Fall of 2002 (Mission 2006) charged students with developing a strategy for monitoring and preserving the Amazon Rainforest. As in previous years, the students in Mission 2006 described their final design in a content-rich web site and an oral presentation in front of a panel of international experts. Mission 2007 was focused on Arctic National Wildlife Refuge (ANWR), Mission 2008 - Galapagos, Mission 2009 -Tsunamis and Mission 2010 - Saving N'awlinz, Mission 2011 - Saving our oceans, Mission 2012 - Clean water-assuring clean fresh water for western North America, Mission 2013 - CO2 sequestration, and Mission 2014, Feeding the World, and Mission 2015, Whole Earth Triage - Securing the future of biodiversity. |
 |
 |